DEFINITION
Specific
gravity G is defined as the ratio of the weight of an equal volume of
distilled water at that temperature both weights taken in air.
APPARATUS REQUIRED
1. Density bottle of 50 ml with stopper having capillary hole.
2. Balance to weigh the materials (accuracy 10gm).
3. Wash bottle with distilled water.
4. Alcohol and ether.
1.
Clean and dry the density bottle
2.
Weigh the empty bottle with stopper (W1)
3.
Take about 10 to 20 gm of oven soil sample which is cooled in a desiccator.
Transfer it to the bottle. Find the weight of the bottle and soil (W2).
4.
Put 10ml of distilled water in the bottle to allow the soil to soak completely.
Leave it for about 2 hours.
5.
Again fill the bottle completely with distilled water put the stopper and keep
the bottle
under constant temperature water baths (Tx0 ).
6.
Take the bottle outside and wipe it clean and dry note. Now determine the weight
of the bottle and the contents (W3).
7.
Now empty the bottle and thoroughly clean it. Fill the bottle with only
disttiled water and weigh it. Let it be W4 at temperature (Tx0
C).
8.
Repeat the same process for 2 to 3 times, to take the average reading of it.
|
S.
No. |
Observation
Number |
1 |
2 |
3 |
|
1 2 3 4 |
Weight
of density bottle (W1 g) Weight
of density bottle + dry soil (W2 g) Weight
of bottle + dry soil + water at temperature T x0
C (W3 g) Weight
of bottle + water (W4 g) at temperature Tx0
C |
|
|
|
|
|
Specific
gravity G at Tx0 C |
|
|
|
|
|
Average
specific gravity at Tx0 C |
|
|
|
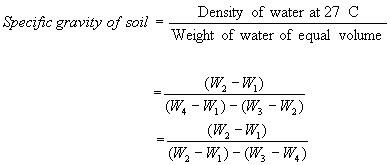
Unless or otherwise specified specific gravity values reported shall be based on water at 270C. So the specific gravity at 270C = K´Sp. gravity at Tx0C.

The specific gravity of the soil particles lie with in the range of 2.65 to 2.85. Soils containing organic matter and porous particles may have specific gravity values below 2.0. Soils having heavy substances may have values above 3.0.