Live Cell Multiphoton Imaging: Principles & Experiments
OBJECTIVE: Femtosecond laser
pulses, when focused onto a fluorophore, can generate two-photon fluorescence
from the focus only due to its very high peak power. Using this property,
femtosecond laser pulses are used in multiphoton microscopy. It has better
depth resolution as well as less toxicity compared to the confocal microscopy
as here a red shifted light is used for excitation.
THEORY: In multiphoton
microscope, where mainly two-photon fluorescence is generated and detected,
unlike the confocal microscope, no confocal aperture is needed as the
two-photon fluorescence is generated only from the focal spot. Moreover, here
we can simultaneously excite a whole range of fluorophores using a single laser
unlike the confocal microscope as fluorophores have very wide two-photon
absorption band compared to its single photon absorption band. The small
difference between the multiphoton and confocal microscope is that in the
former case we do not need the pinhole, but we need filter (s) to separate the
fluorescence coming from the various fluorophores.
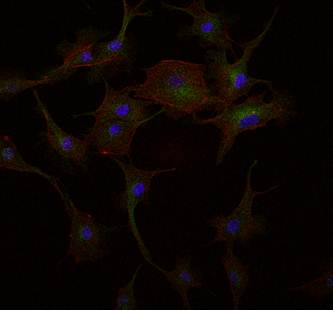
Image
of a BPAE tissue under two-photon excitation
INSTRUMENTS REQUIRED:
- Excitation
Pulsed Laser
- Microscope
- Microscope
Objective
- Fluorescent
Sample
- Dichroic
Mirror
- Filters
- Detector
SOFTWARE REQUIRED:
- FLUOVIEW
Note: For user
operation and usage no specific software needed.
EXPERIMENT PROCEDURE:
1.
Turn
on the excitation Laser.
2.
Turn
on the ‘ML’ button to convert it to the femtosecond pulse Laser.
3.
Place
a drop of immersion oil on the oil immersion microscope objective of a specific
magnification
4.
Place
the microscopic slide of stained live cell on the sample plane.
5. Tighten
it with the clamps.
6.
Turn
on the UV lamp.
7.
Move
the objective such that the oil placed on it touches the bottom side of the
slide.
8.
Let
the UV light pass through the objective and get focused on the sample (i.e.,
microscopic slide) and see the fluorescence coming from the stained live cell
through the microscope eye piece.
9.
Focus
the sample seeing the fluorescence of the cells.
10. Block the UV lamp.
11. Turn on the FLUOVIEW
software for image collection.
12. Fix the power of the
excitation Laser beam.
13. Let the excitation Laser
beam pass through the microscope objective.
14. Set the value of
confocal aperture to its maximum value as multiphoton microscope does not need
any confocal aperture.
15. Place proper
fluorescence filter (i.e., band pass filter) for selecting the fluorescence
coming from the specific region of the cells (e.g., nucleus, microtubules
etc.).
16. Turn on the Photo
Multiplier Tube (PMT) detector to collect the fluorescence.
17. Increase the PMT
voltage to have a good signal.
18. Select the area of the
cell to be selected.
19. Click on the ‘scan
once’ button getting the image of the live cell sample.
20. Save the image by
clicking the buttons in the following order: File I/O =>
Save Image as and then select the file type (.TIFF or .BMP) and where to save
the image and then click the ‘save’ button to save the image.