There are certain situations when two phases of a pure substance coexist in equilibrium. As a commonly used substance water may be taken up to demonstrate the basic principles involved. However, all pure substances exhibit the same general behaviour.
We shall remember the following definitions:
Saturated State: A state at which a phase change begins or ends
Saturation Temperature: Temperature at which phase change (liquid-vapour) begins or ends at a given pressure
Saturation Pressure: It is the pressure at which phase changebegins or ends at a specified temperature.
Saturated Liquid: It is the substance at 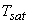 which is fully liquid (no-vapour). which is fully liquid (no-vapour).
Saturated Vapour: It is the substance at 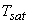 which is fully vapour (no-liquid). which is fully vapour (no-liquid).
Subcooled liquid: If the temperature of the liquid (T) is less then 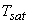 then the liquid is called sub-cooled liquid. then the liquid is called sub-cooled liquid.
Superheated vapour: If the temperature of the vapour (T) is greater than 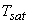 then the vapour is called superheated vapour. then the vapour is called superheated vapour.
|