Second law analysis of a control volume
It was shown that the change in entropy of a system is given by
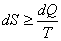 |
(24.12) |
Where the equality sign holds good for the reversible processes and the inequality sign holds for all irreversible processes. This can be expressed as
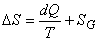 |
(24.13) |
Where 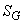 represents entropy generation in the system and it cannot take a negative value. represents entropy generation in the system and it cannot take a negative value.
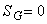 for a reversible process. for a reversible process.
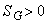 for an irreversible process. for an irreversible process.
Consider a control volume through which material flows (Figure 24.2)
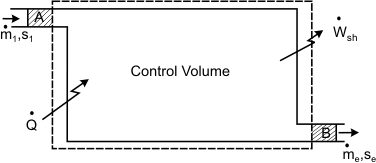
Figure 24.2 Let us identify a system such that its mass remains constant during a given process. At time t, the system constitutes both the mass inside the control volume and the mass about the enter the control volume during a small interval dt (that is, the mass occupying the region A). At time (t+dt), the system is the mass inside the control volume as well as the mass in region B. During the time interval dt, the system undergoes a change in configuration and receives energy as heat 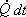 from the surroundings. from the surroundings.
At time t, the entropy of the system
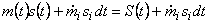 |
(24.14) |
At time 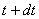 , the entropy of the system , the entropy of the system
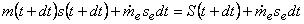 |
(24.15) |
Then,
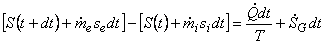 |
(24.16) |
or,
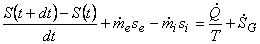 |
(24.17) |
or,
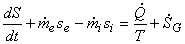 |
(24.18) |
This can be rearranged as
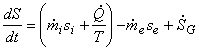 |
(24.19) |
In words:
The rate of accumulation of entropy = Rate of inflow of entropy
- Rate of outflow of entropy + Rate of generation of entropy
|