Example
In a desalination plant, drinking water is produced by condensing steam in a heat exchanger. Steam at 0.1 MPa and 1500C enters the heat exchanger. Shown in fig at the rate of 10000 kg/h and emerges as a liquid at 400C. Cooling water enters the heat exchanger at 250C and leaves at 350C. Estimate the loss in availability in the heat exchange process. The ambient temperature is 300K
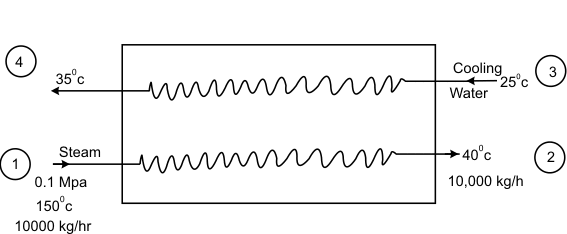
Figure 26.1
First law: 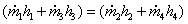
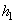 = 2775.8 kJ/ kg --------------- = 2775.8 kJ/ kg --------------- 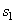 = 7.5984 kJ/ kg K = 7.5984 kJ/ kg K
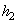 = 167.45 kJ/ kg --------------- = 167.45 kJ/ kg --------------- 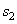 = 0.5721 kJ/ kg K = 0.5721 kJ/ kg K
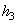 = 104.77 kJ/ kg --------------- = 104.77 kJ/ kg --------------- 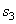 = 0.3670 kJ/ kg K = 0.3670 kJ/ kg K
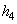 = 146.56 kJ/ kg --------------- = 146.56 kJ/ kg --------------- 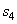 = 0.5049 kJ/ kg K = 0.5049 kJ/ kg K
104 (2775.8) + 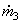 (104.77) = 10 4 (167.45) + (104.77) = 10 4 (167.45) + 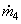 (146.56) (146.56)
or 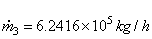
Change in availability of system
Change in availability of cooling water
Net change in availability
But 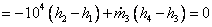
Hence net change in the availability
Therefore the loss in availability 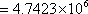 kJ kJ
|