LECTURE 11
Control-Volume
Analysis
Control
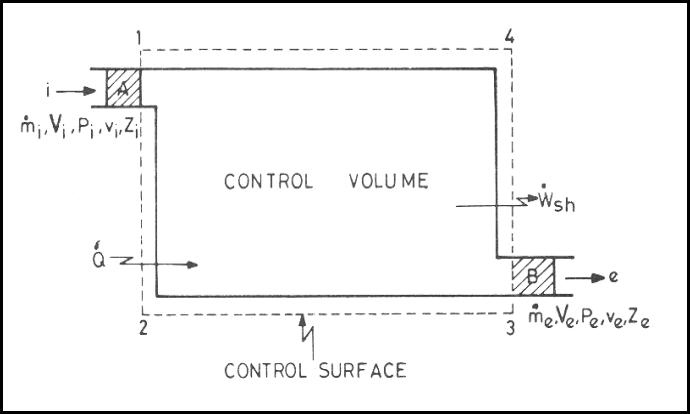
volume is a volume in space of special interest for particular analysis.
The
surface of the control volume is referred as a control surface and is a closed
surface.
The
surface is defined with relative to a coordinate system that may be fixed,
moving or rotating.
Mass,
heat and work can cross the control surface and mass and properties can change
with time within the control volume.
Examples:
turbines, compressors, nozzle, diffuser, pumps, heat exchanger, reactors, a
thrust-producing device, and combinations of these.
First
law of thermodynamics for a continuous system
Let the continuous system be in state 1 at time
t and after a differential time dt, let it be in the state 2. The change in the
energy of the continuous system is,
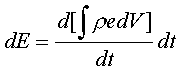
Now,
dE =
dQ – dW
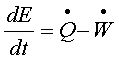 or,
or,
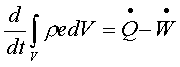
First
law of thermodynamics to a control volume
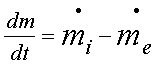
Or
[Rate of accumulation of mass inside the
control volume] = [Rate of mass entering the control volume at
inlet] – [Rate of mass leaving the control volume at exit]
The above
is commonly known as continuity equation.
We should
identify a definite quantity of matter which remains constant as the matter
flows. For this purpose, let the boundary of the system include all matter
inside the control volume and that which is about to enter the control volume
during the differential time interval dt.
At
time t, the system is defined as the
mass contained in the control volume and the mass in region A which is about to
enter the control volume in a differential time dt.
At
time t+dt, the system is defined as the mass contained in the control volume
and the mass in region B.
Therefore,
during the differential time dt, the system configuration undergoes a change.
Mass
contained in region A = ![]() dt
dt
Mass
contained in region B = ![]() dt
dt
From
mass balance,
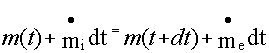
The
work done as the mass enters the control volume = -Pivi![]() dt
dt
The
work done by mass exiting the control volume =
Peve![]() dt
dt
Energy
of the system at time t = E(t) +![]() eidt
eidt
Energy of the system at time (t+dt) =
E(t+dt) + ee ![]() dt
dt
Energy transferred as heat to the system = ![]() dt
dt
Shaft work done by the system = 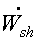 dt
dt
From
the first law,
![]() [E(t+dt) + ee
[E(t+dt) + ee ![]() dt] – [E(t) +
dt] – [E(t) +![]() eidt ] =
eidt ] = ![]() dt -
dt - ![]() dt – ( Peve
dt – ( Peve![]() -Pivi
-Pivi![]() )dt
)dt
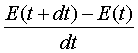
![]()
![]() ( ee + Peve)-
( ee + Peve)-
![]() (ei + Pivi)=
(ei + Pivi)= ![]() -
- ![]() -
-
or,
![]() (he+Ve2/2
+ gZe) -
(he+Ve2/2
+ gZe) - ![]() (hi+Vi2/2 + gZi)
=
(hi+Vi2/2 + gZi)
= ![]() -
- ![]() - dE/dt
- dE/dt
where,
he = ue + Peve, hi = ui + Pivi
Or, Rate
of energy accumulation = rate of energy inflow – rate of energy outflow
Steady state flow process
Assumptions:
![]()
![]()
![]() are equal
are equal
The
state of matter at the inlet, exit and at any given point inside the control
volume does not change with respect to time.
dE / dt = 0
The rate
of energy transfers across the control surface is constant.
![]() (he+Ve2/2 + gZe) - (hi+Vi2/2
+ gZi) =(
(he+Ve2/2 + gZe) - (hi+Vi2/2
+ gZi) =( ![]() -
- ![]() )/
)/