LECTURE 13
A systematic approach to problem solving
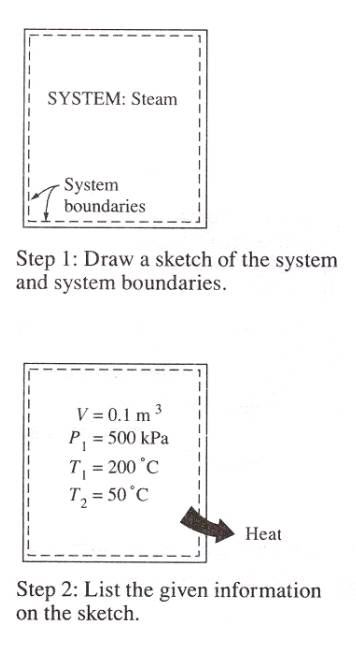
Step 1. Identify the
system and draw a sketch of it. The system that is about to be analyzed should
be identified on the sketch by drawing its boundaries using the dashed lines.
Step 2. List the given
information on the sketch. Heat and work interactions if any should also be
indicated on the sketch with proper directions.
Step 3. State any assumptions:
The simplifying assumptions
that are made to solve a problem should be stated and fully justified.
Commonly made assumptions:
(a)
Assuming process to be quasi-equilibrium
(b) Neglecting PE
and KE
(c) Treating gas as
ideal
(d) Neglecting heat
transfer from insulated systems.
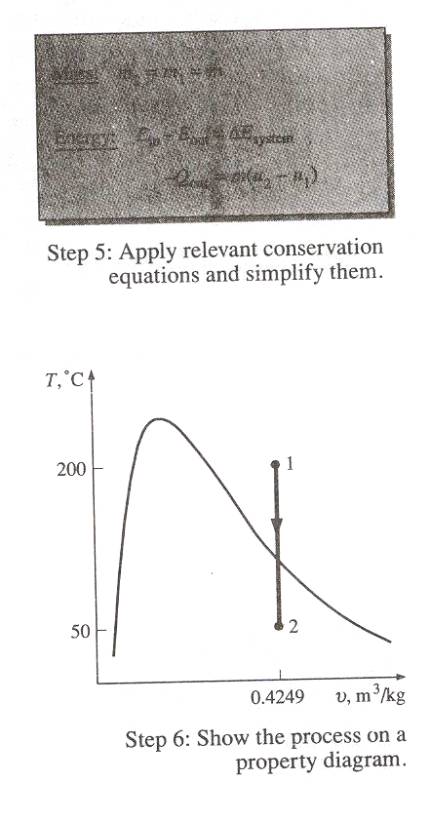
Step 5. Apply the
conservation equations.
Step 6. Draw a process
diagram.
Determine the required
properties and unknowns.
Problem # 1 A 0.1 m3 rigid tank contains steam
initially at 500 kPa and 200oC. The steam is now allowed to cool
until the temperature drops to 50oC. Determine the amount of heat transfer
during this process and the final pressure in the tank.
State 1: P1
= 500kPa, T1 = 200oC
v1
= 0.4249 m3/kg, u1 = 2642.9 kJ/kg
State 2: v2
= v1 = 0.4269 m3/kg
T2
= 50oC à vf = 0.001m3/kg
vg= 12.03 m3/kg
uf = 209.32 kJ/kg
ug = 2443.5 kJ/kg
P2 = Psat @50oc = 12.349
kPa
v2 = vf
+ x2vfg
0.4249 = 0.001 + x2(12.03
= 0.001)
x2 = 0.0352
u2 = uf +x2ug
= 209.32 +(0.0352)(2443.5 – 209.32)
= 288.0 kJ/kg
m = V/u = (0.1 m3/kg)/(0.4249
m3/kg)
= 0.235 kg
-Qout = DU = m(u2 – u1)
Qout = m(u1
– u2)
= (0.235)(2642.9 – 288)
= 553.4 kg
Problem # 2 A piston/cylinder contains 50 kg of water at 200 kPa
with a volume of 0.1 m3 . Stop in the cylinder is placed to restrict
the enclosed volume to 0.5 m3. The water is now heated until the
piston reaches the stops. Find the necessary heat transfer.
At 200 kPa,
vf = 0.001061 m3/kg
vfg = 0.88467 m3/kg
hf = 504.68 kJ/kg
hfg = 2201.96 kJ/kg
This is a constant pressure
process. Hence,
Q = DH
The specific volume
initially,
vi = 0.1 /50 = 0.002
m3/kg
v = vf + x vfg
= 0.001061 + x
(0.88467)
Therefore, x = (0.002 – 0.001061) / 0.88467
=
0.001061
h = hf + x hfg
= 504.68
+ 0.001061(2201.96)
=
507.017 kJ/kg
vfinal = 0.5 /50 = 0.01 m3/kg
v = vf + x vfg
Therefore, x = (0.01 – 0.001061) /
0.88467
= 0.01
hfinal = 504.68 + 0.01(2201.96)
= 526.69 kJ/kg
Q = DH = 50 (526.69 - 507.017)
= 983.65 kJ/kg
Problem # 3 A rigid insulated tank is
separated into two rooms by a stiff plate. Room A of 0.5 m3 contains
air at 250 kPa, 300 K and room B of 1 m3 has air at 150 kPa, 1000 K.
The plate is removed and the air comes to a uniform state without any heat
transfer. Find the final pressure and
temperature.
The system comprises of room A and B together. This is a constant internal energy process as there is no heat and work exchange with the surroundings.
mA = PAVA
/ RTA
= (250 x 1000 x 0.5) / (287 x 300)
= 1.452 kg
mB = PBVB
/ RTB
= (150 x 1000 x 1.0) / (287
x 1000)
= 0.523 kg
DUA + DUB = 0
Let Tf be the final temperature at equilibrium
mA (Tf – 300) + mB (Tf –
1000) = 0
1.452 (Tf – 300) + 0.523 (Tf –
1000) = 0
Tf = 485.37
K
Pf = (1.452 + 0.523) x 287 x 485.37 / 1.5
= 183.41 kPa

Problem # 4 A piston / cylinder assembly contains 0.1m3 of
superheated steam at 10 bar and 400oC. If the steam is allowed to
expand reversibly and adiabatically to a pressure of 3 bar, calculate the work
done by the steam.
At 10 bar and 400oC,
v = 0.3065 m3/kg
h = 3264.4 kJ/kg
s = 7.4665 kJ/kg K
At 3 bar,
sg =
6.9909 kJ/kg K
This is an isentropic
process as initial entropy value is greater than sg at 3 bar, the
steam is superheated at the end of the process.
At 3 bar and 200oC,
s = 7.3119 kJ/kg K and
at 300oC, s =
7.7034 kJ/kg K
therefore, the final state
is having a temperature between 200oC and 300oC.
Equating si = sfinal,
Find the enthalpy and
specific volume by interpolation. Then calculate ui and ufinal.
The work done = DU = m(ui – ufinal)