LECTURE 14
Enthalpy of a compressed liquid
Determine the enthalpy of
water at 100o C and 15 MPa (a) by using compressed liquid tables,
(b) by approximating it as a saturated liquid, and (c) by using the correction
factor.
At 100oC, the saturation
pressure of water is 101.35 kPa, and since P>Psat, the water
exists as a compressed liquid at the specified state.
(a) from the
compressed liquid tables,
P = 15 Mpa, T = 100oC, h = 430.28 kJ/kg
This is the exact value.
(b) Approximating the
compresses liquid as a saturated liquid at 100oC, as is commonly
done, we obtain
h = hf@100 C = 419.04 kJ/kg
This value is in error by
about 2.6 percent.
(c) From equation
h@ P,T = hf@T + vf@T
(P – Psat)
= 419.04 + 0.001(15000 – 101.35) kJ/kg
= 434.60 kJ/kg
Problem # 1 (Nozzle)
Nitrogen gas flows into a convergent nozzle at 200 kPa, 400 K and very low velocity. It flows out of the nozzle at 100 kPa, 330 K. If the nozzle is insulated, find the exit velocity.
Vi = 0
Adiabatic nozzle
The SSSF equation:
Ve2/2 = (hi – he) = Cp(Ti – Te)
= {gRu/M(g-1)} (Ti – Te)
= {1.4 * 8314/(28*0.4)}(400-330)
= 72747.5 m2/s2
We get, Ve = 381.44 m/s
Problem # 2 (Diffuser)
Air at 10o C and
80 kPa enters the diffuser of a jet engine steadily with a velocity of 200 m/s.
The inlet area of the diffuser is 0.4 m2. The air leaves the
diffuser with a velocity that is very small compared with the inlet velocity.
Determine (a) the mass flow rate of the air and (b) the temperature of the air
leaving the diffuser.

Solution:
Assumptions: This is a steady
flow process. Air is an ideal gas. The potential energy change is zero. Kinetic
energy at diffuser exit is negligible. There are no work interactions. Heat transfer
is negligible.
To determine the mass flow
rate, we need the specific volume of air.
v1 = RT1/
P1 = 0.287 * 283 / 80 = 1.015 m3/kg
m = 1/v1(V1A1)
= (200 * 0.4)/ 1.015 = 78.8 kg/s
For steady flow, mass flow through the diffuser is constant.
(b) (h1 + V12/2)
= (h2 + V22/2) (since Q = 0, W = 0, and DPE = 0)
h2 = h1
– (V22- V12)/2
The exit velocity of a diffuser is very small and therefore neglected.
h2 = h1 + V12/2
T2 = T1
+ V12/2Cp
T2 = 283 + 2002/(2*1004)
= 302.92 K
Compressing air by a compressor
Air at 100 kPa and 280 K is compressed steadily to 600 kPa and 400 K. The mass flow rate of air is 0.02 kg/s and a heat loss of 16 kJ/kg occurs during the process. Assuming the changes in KE and PE are negligible, determine the necessary power input to the compressor.
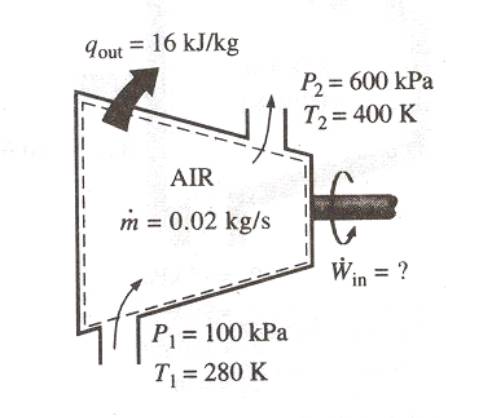
We take the compressor as the system. This is a control volume since
the mass crosses the system boundary during the process. Heat is lost from the system
and work is supplied to the system.
With similar assumptions as in the diffuser problem,
w = q + (h2 – h1)
The input power = m (q +
(h2 – h1))
= 0.02 (16 + (1.004*(400 –
280)))
= 2.73 kW
Power generation by a steam turbine
The power output of an adiabatic steam turbine is 5 MW, and the inlet
and exit conditions of the steam are as indicated in the figure.
(a)
Compare the magnitude of Dh, DKE, and DPE
(b)
Determine the work done per unit mass of the steam flowing through the
turbine
(c)
Calculate the mass flow rate of the steam.
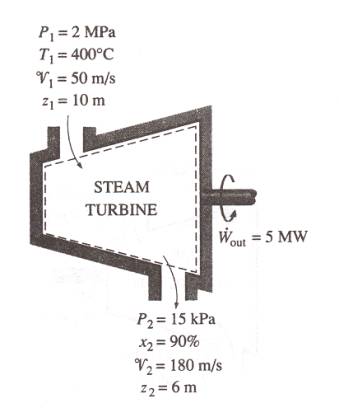
We take the turbine as a system. The control volume is shown in the figure. The system, the inlet and exit velocities do work and elevations are given and thus the kinetic and potential energies are to be considered.
At the inlet, the steam is
in superheated vapor state.
h1 = 3247.6 kJ/kg.
At the turbine exit, we have a saturated liquid-vapor mixture at 15 kPa pressure. The enthalpy at this state is
h2 = hf + x2hfg
= 225.94 + 0.9 * 2373.1
= 2361.73 kJ/kg
Dh = h2
– h1
= 2361.73 – 3247.6 =
-885.87 kJ/kg
Dke = (V22-
V12)/2 = (1802-502)/2*1000
= 14.95 kJ/kg
DPe = g(Z2-Z1)
= 9.807 * (6 –10)/1000
= -0.04 kJ/kg
wout = -[( h2
– h1) +(V22- V12)/2 +
g(Z2-Z1)]
= -[-885.87 + 14.95 – 0.04]
= 870.96 kJ/kg
(d)
The required mass flow rate for a 5MW power output is 5000/870.96 = 5.74 kg/s