Molecules of a system pass energy in various forms such as sensible and latent energy (associated with change of state), chemical energy (associated with the molecular structure), and nuclear energy (associated with the atomic energy).
During a chemical reaction, some chemical bonds that bind the atoms into molecules are broken, and new ones are formed.
The chemical energy associated with these bonds, in general, is different for the reactants and the products.
During a chemical reaction:
DEsystem = DEstate + DEchem
When the products formed during a chemical reaction exit the reaction chamber at the inlet state of the reactants, we have,
DEstate = 0
Energy change of system is only due to change in chemical composition.
To have a common reference state for all substances, a reference state 25oC and 1 atm. Is taken as a standard reference state. Property value at the standard reference state is indicated by a superscript “o” (such as ho and uo).
Enthalpy of reaction
It is the difference between the enthalpy of the products at the specified state and the enthalpy of the reactants at the same state for a complete reaction.
DHR = -393,520 kJ/kmol C
![]()
![]()
![]()
 1 kmol C
1 kmol C
![]() 1kmolO2 1kmol
CO2
1kmolO2 1kmol
CO2
25oC,1atm
Enthalpy of combustion hc, which represents the amount of heat released during a steady-flow combustion process when 1 kmol (or 1kg) of fuel is burned completely at a specified temperature and pressure.
A more fundamental property to represent the chemical energy of an element or compound at a reference state is enthalpy of formation.
It is defined as enthalpy of a substance at a specified state due to its chemical composition.
The enthalpy of formation of all stable elements (such as O2, N2, H2 and C) is assigned a value of zero at the standard state of 25oC and 1atm.
For all stable elements DHof298 is zero. The stable form of an element is simply the chemically stable form of that element at 25oC and 1 atm. The diatomic form (N2) for example is the stable form at 25oC and 1 atm and not monoatomic nitrogen N.
DHf = Q = -393,520 kJ/kmol CO2
![]()
![]()
![]()
 1 kmol C
1 kmol C
![]() 1kmolO2 1kmol
CO2
1kmolO2 1kmol
CO2
25oC,1atm 25oC,1atm
The standard heats of formation for common compounds are available in tabulated form.
Example:
Calculate the standard heat DHof298 for
the following reaction
C5H12
(g) + 8O2(g) à 5CO2 (g) + 6H2O
(g)
Solution:
The standard heats of
formation DHof298
of the chemical species involved in the reaction are.
CO2(g) : - 393.51 kJ; H2O(g):
-241.82 kJ
C5H12(g):
-146.76 kJ; O2(g): 0 kJ
The following combination of
the formation reactions gives the desired reaction.
C5H12(g) à 5C(s) + 6H2(g) DHo298
= 146.76 kJ
5{C(s) + O2(g) àCO2(g)}
DHo298
= 5(-393.51)kJ
6{H2(g)
+ ½ O2(g) àH2O(g)} DHo298 = 6(-241.82)kJ
C5H12
(g) + 8O2(g) à 5CO2 (g) + 6H2O
(g)
DHo298 = -3271.71 kJ
Example:
Estimate the standard heat DHof298
of the following reaction:
C5H12
(g) + 8O2(g) à 5CO2 (g) + 6H2O
(l)
Assume that the latent heat
of vaporization of water at 298.15 K is 2442.6 kJ/kg
Solution:
The standard heat DHo298 of the reaction
C5H12
(g) + 8O2(g) à 5CO2 (g) + 6H2O
(g)
DHo298 = -3271.71 kJ
6{H2O(g) à H2O}(l) DHo298
= 6(-43.97)kJ
The latent heat of
vaporization of water =
(2442.6 x 18)/1000 = 43.97
kJ/mol
Hence the required
DHo298 = -3271.71 + 6(-43.97)
= -3535.53 kJ
Standard heat of combustion
A combustion reaction is defined as a reaction between the element or compound and oxygen to form specified combustion products.
The heat of reaction for a
combustion reaction when the reactants and products are in their respective
standard states is called the standard heat of combustion.
Another term commonly used
is “heating values” of the fuel. If
the water in the products is in the liquid form, it is called higher
heating value and if it is in vapor form, it is called lower heating
value.
Example:
Estimate the gross heating
value and net heating value of pentane if the reactants and products are at 25oC.
Solution:
The combustion reaction is given by
C5H12
(g) + 8O2(g) à 5CO2 (g) + 6H2O
(g)
The standard heat of the
above reaction is estimated as
DHo298 = -3271.71 kJ per mol of pentane.
Therefore,
Net heating value = lower
heating value
= -DHoC
= 3271.71 kJ
If the water in the products
is in the liquid state, the combustion reaction is given by
C5H12
(g) + 8O2(g) à 5CO2 (g) + 6H2O
(l)
The standard heat of this reaction is
DHo298
= -3535.53 kJ per mol of pentane
Gross heating value or
higher heating value,
- DHoC =
3535.53 kJ
Effect of temperature on the standard heat of reaction
The standard heat of
reaction at temperature T can be estimated in the following steps:
Reactants at Desired change Products at
temperature, T à temperature, T
![]() DHoT Step 3
DHoT Step 3
![]() DHoR
DHoP
Heating Step1
DHoR
DHoP
Heating Step1
cooling
Step 2
Reactants at à Products at
T = 298.15 K DHo298 T = 298.15 K
1. The reactants in their standard states are cooled at constant pressure (0.1 MPa) from T to 298.15 K. The change in the enthalpy associated with this process is
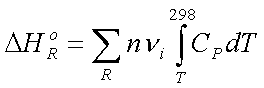
2.
The reaction is allowed to proceed at 298.15 K. The change in enthalpy
associated with this process is given by DHo298.
3.
The products in their standard states are heated from 298.15 K to T.
The change in enthalpy associated with this process is given by
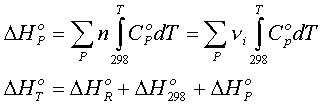
Adiabatic
flame temperature
The chemical energy released during a combustion process is either lost as heat to the surroundings or is used internally to raise the temperature of combustion products.
If no loss to the
surrounding occurs, the temperature of the products will reach a maximum, which
is called the adiabatic flame temperature.
The adiabatic flame temperature depends on:
1. state of the
reactants
2. the degree of
composition of reaction
3. the amount of air
used
The adiabatic flame
temperature is maximum when the complete combustion occurs with theoretical
amount of air.
In combustion chambers, the
highest temperature to which a material can be exposed is limited by
metallurgical considerations. Hence, the value of adiabatic flame temperature
is an important consideration for combustion chambers. Actual temperatures are
usually lower than the adiabatic flame temperature.
Example:
Estimate the adiabatic flame
temperature that can be reached by the combustion of n-Pentane with 25 percent
excess air. Both the fuel and air enters the burner at 25oC. Assume
complete combustion.
Solution:
The first law of thermodynamics for a steady flow process, ignoring the changes in the kinetic energy and potential energy is given by:
He – Hi
= Q = 0 or DH = 0
The burner is adiabatic: Q =
0, Wsh = 0
C5H12(g)
+ 10O2(g) à 5CO2(g)
+ 6H2O(g) +2O2(g) + 37.6N2(g)

![]() + 37.6N2
+ 37.6N2
298 K DH = 0 DHP
5CO2(g) +6H2O(g)
+ 2O2(g) + 37.6N2(g)
(T)
The process that occurs in
the reactor can be treated as consisting of two steps. First the reactants are
converted into products at 298 K. The energy change associated with this step
is equal to DHo298
. In the second step, the products are raised to temperature, T. The energy
change associated with this step is equal to DHP and is given
by
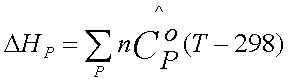
The energy transferred as
heat to the surroundings is Q which is equal to the overall change in the
enthalpy (DHo298
+DHoP).
Q =DHo298 +DHoP = 0
DHo298
= -3271.71 kJ
The average molar heat
capacities of CO2, H2O, O2 and N2
are 62.75 J/mol K, 52.96 J/mol K, 38.67 J/mol K and 37.13 j/mol K,
respectively.
Therefore,
DHoP =
(5 x 62.75 + 6 x 52.96 + 2 x 38.67 + 37.6 x 37.13) x 10-3 (T-298)
Hence,
-3271.71 + (5 x 62.75 + 6 x
52.96 + 2 x 38.67 + 37.6 x 37.13) x 10-3 (T-298) = 0
or,
T = 1852.3 K
Example:
An internal combustion
engine uses octane as fuel. The air and fuel vapour mixture enter the engine at
25°C and 0.1 MPa and
the engine uses 120 percent theoretical air. Supposing 75% of the fuel’s carbon
is converted into CO2 and the rest is converted to CO, and the
combustion products leave the engine at 800K, calculate the amount of energy
transferred as heat to the engine (per kg of fuel).
If the molar specific heat
capacities in the ideal gas state for the reactants and products are expressed
as a function of temperature by equations of the form,
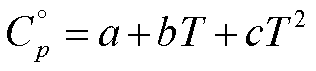
an analytical expression for
![]() as a function of temperature can be obtained as shown below.
as a function of temperature can be obtained as shown below.
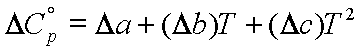
where
![]()
substituting,
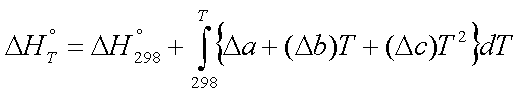
or,
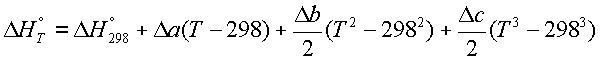
or,
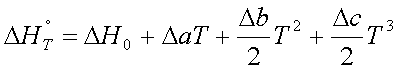
where, all the constants are
lumped together in ![]()
Solution:
![]()
![]()
![]() Reactants(298K) Products(298K)
Reactants(298K) Products(298K)

![]()
![]()
DHp DH Products(800K)
![]()
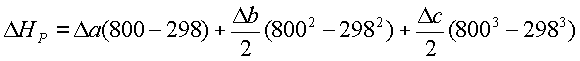
(refer table 16.3 of
Y.V.C.Rao for constants)
Da=(6*5.457+2*3.376+9*3.47+3.5*3.639+56.4*3.28)*8.314=2231.914
Db=(6*1.045+2*0.557+9*1.45+3.5*0.506+56.4*0.593)*10-3*8.314=0.4627
Dc=0
DH=-4549.75+2231.914*10-3*(800-298)+
0.4627*10-3*(8002-2982)/2
or DH=-3301.81 kJ
Molar mass of C8H18=114kg/kmol
Hence DH=-28.96kJ/kg
fuel
Energy transferred as heat=28.96kJ