Limitations
of First Law
First law is a statement of
conservation of energy principle. Satisfaction of first law alone does not
ensure that the process will actually take place.
Examples:
1.
A cup of hot coffee left in a cooler room eventually cools off. The
reverse of this process- coffee getting hotter as a result of heat transfer
from a cooler room does not take place.
2.
Consider heating of a room by passage of electric current through an
electric resistor. Transferring of heat from room will not cause electrical
energy to be generated through the wire.
3.
Consider a paddle-wheel mechanism operated by fall of mass. Potential
energy of mass decreases and internal energy of the fluid increases. Reverse
process does not happen, although this would not violate first law.
4.
Water flows down hill where by potential energy is converted into K.E.
Reverse of this process does not occur in nature.
Conclusion:
Processes proceed in a
certain direction and not in the reverse direction. The first law places no
restriction on direction.
A process will not occur
unless it satisfies both the first and second laws of thermodynamics.
Second law not only
identifies the direction of process, it also asserts that energy has quality as
well as quantity.
Thermal Reservoir
A thermal reservoir is a
large system (very high mass x specific heat value) from which a quantity of
energy can be absorbed or added as heat without changing its temperature. The
atmosphere and sea are examples of thermal reservoirs.
Any physical body whose
thermal energy capacity is large relative to the amount of energy it supplies
or absorbs can be modeled as a thermal reservoir.
A reservoir that supplies
energy in the form of heat is called a source and one that absorbs energy in
the form of heat is called a sink.
Heat Engine
It is a cyclically operating
device which absorbs energy as heat from a high temperature reservoir, converts
part of the energy into work and rejects the rest of the energy as heat to a
thermal reservoir at low temperature.
The working fluid is a
substance, which absorbs energy as heat from a source, and rejects energy as
heat to a sink.
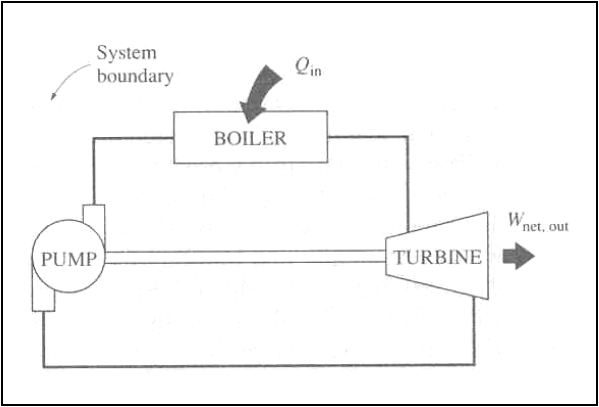
Thermal Power Plant
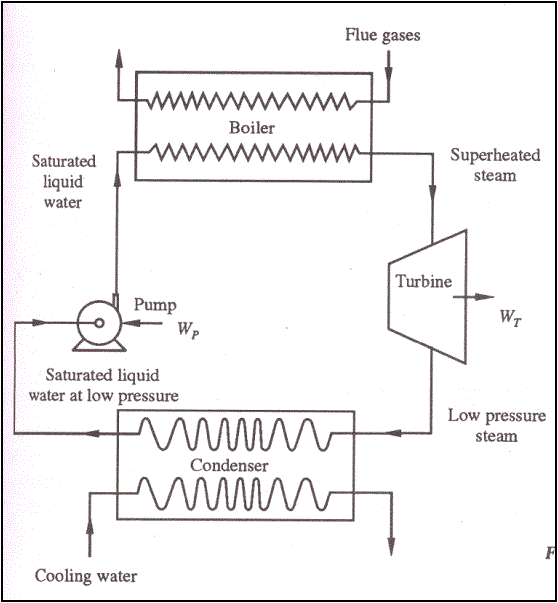
Working Fluid ------- Water
Q1 – Heat
received from hot gases
WT – Shaft work
by turbine
Q2 – Heat
rejected to cooling water in condenser
WP – Work done on
the pump
Wnet=WT-WP
W = Q1 – Q2
Thermal Efficiency,
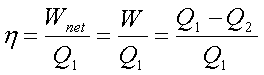
Schematic representation of Heat Engine
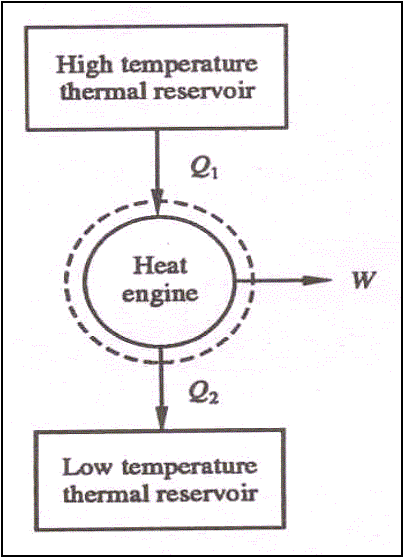
Schematic representation of Refrigerator and Heat pump.
QL – Heat
absorbed from low temperature thermal reservoir
QH – Heat
rejected to a high temperature thermal reservoir when work (W) is done on it.
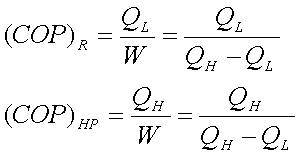
In a reversible, isothermal
expansion of an ideal gas, all the energy absorbed as heat by the system is
converted completely into work. However this cannot produce work continuously
(not a cycle).
Single reservoir heat engine
(1 T engine) is not possible.
Second Law of Thermodynamics
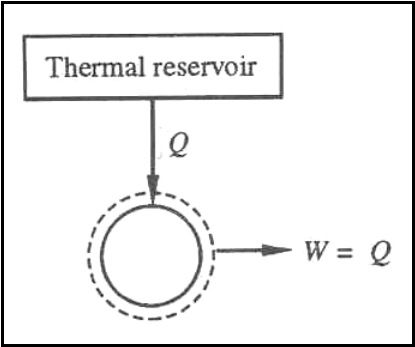
Kelvin-Planck Statement: - It is impossible
to devise a cyclically operating device, which produces no other effect than
the extraction of heat from a single thermal reservoir and delivers an
equivalent amount of work.
Heat engine with single
thermal reservoir is not possible.
For a 1-T engine the thermal
efficiency h=W/Q=1. No heat
engine can have efficiency equal to unity.
Clausius Statement: - It is
impossible to construct a device that operates in a cycle and produces no
effect other than the transfer of heat from a lower-temperature body to
higher-temperature body.
Equivalence of
the two statements
To prove that violation of
the Kelvin-Planck Statement leads to a violation of the Clausius Statement, let
us assume that Kelvin-Planck statement is incorrect.
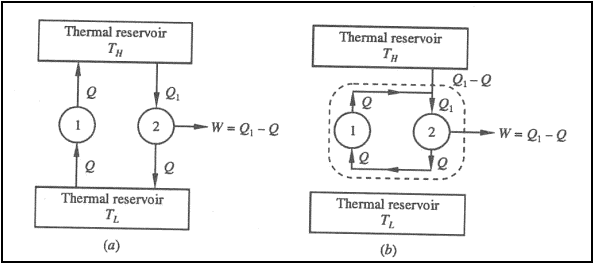
Consider a cyclically
working device 1, which absorbs energy Q1 as heat from a thermal
reservoir at TH. Equivalent amount of work W(W=Q1) is
performed.
Consider another device 2
operating as a cycle, which absorbs energy QL as heat from a low
temperature thermal reservoir at TL and rejects energy QH (QH=QL+W). Such a
device does not violate Clausius statement.
If the two devices are now
combined, the combined device (enclosed by the dotted boundary) transfers heat
QL from the low temperature reservoir at TL to a high
temperature reservoir at TH with out receiving any aid from an
external agent, which is the violation of the Clausius statement.
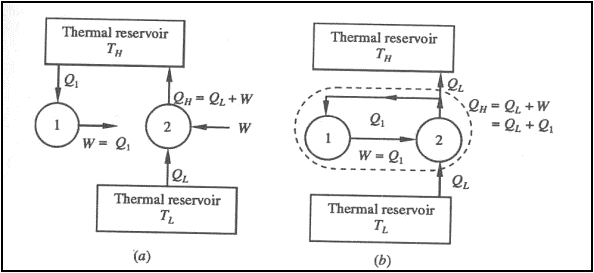
Likewise let us assume that
the Clausius statement is incorrect. So we have a device 1, cyclically working
transferring heat Q from a low temperature reservoir at TL to a high temperature thermal reservoir at TH
. Consider another device 2, which absorbs heat Q1 from a high
temperature reservoir at TH does work W and rejects energy Q as heat
tot the low temperature reservoir at TL as shown in figure.
If the two devices are
combined (shown in figure by a dotted enclosure), then the combined device
receives energy (Q1-Q) as heat from a thermal reservoir and delivers
equivalent work (W=Q1-Q) in violation of the Kelvin-Planck statement.
Therefore violation of
Clausius statement leads to the violation of the Kelvin-Planck statement.
Hence, these two statements are equivalent.
Perpetual Motion Machines
A device that violates the First law of thermodynamics (by creating energy) is called a Perpetual Motion Machine of the first kind.
A device that violates the
Second law of thermodynamics is called a Perpetual Motion Machine of the Second
kind.
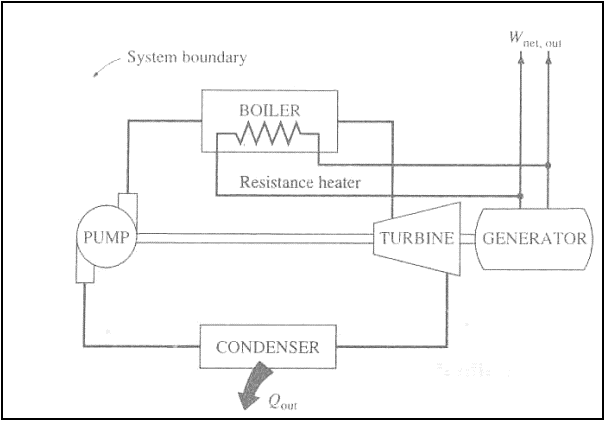
The first device supplies
continuously energy with out receiving it. So this is a system creating energy
and therefore violating the first law.
The second device exchanges
heat with a single reservoir and thus a net amount of work. This need not
violate the first law, but violates the second law and therefore will not work.
Reversible and Irreversible Processes
A process is said to be reversible if both the system and the surroundings can be restored to their respective initial states, by reversing the direction of the process. A reversible process is a process that can be reversed without leaving a trace on the surroundings. Processes that are not reversible are called Irreversible processes.
Irreversibilities
The factors that cause a process to be irreversible are called irreversibilities. Examples:
1. Friction
2. Unrestrained expansion
3. Mixing of two gases
4. Heat transfer across a finite temperature difference
5. Spontaneous chemical reactions
6. Expansion or Compression with finite pressure difference
7. Mixing of matter at different states
Carnot Cycle