The Carnot cycle uses only two thermal reservoirs – one at high temperature T1 and the other at two temperature T2.
If the process undergone by the working fluid during the cycle is to be reversible, the heat transfer must take place with no temperature difference, i.e. it should be isothermal.
The Carnot cycle consists of a reversible isothermal expansion from state 1 to 2, reversible adiabatic expansion from state 2 to 3, a reversible isothermal compression from state 3 to 4 followed by a reversible adiabatic compression to state 1.
The thermal efficiency, h is given by
h = Net work done / Energy
absorbed as heat
During processes 2-3 and
4-1, there is no heat interaction as they are adiabatic.
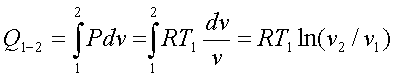
Similarly for the process 3-4,
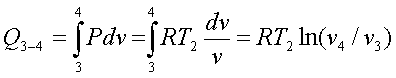
Net heat interaction = Net work done
= RT1ln(v2/v1)
+ RT2ln(v4/v3)
= RT1ln(v2/v1)
- RT2ln(v3/v4)
The processes 2-3 and 4-1 are reversible,
adiabatic and hence
T1v2g-1 = T2v3g-1
Or, v2/v3
= (T2/T1)1/(g-1)
And T2v4g-1 = T1v1g-1
Or, v1/v4
= (T2/T1)1/(g-1)
v2/v3 = v1/v4 or v2/v1
= v3/v4
h = {RT1ln(v2/v1)
- RT2ln(v3/v4)} / RT1ln(v2/v1)
h = (T1
– T2)/T1
= 1- T2/T1
The Carnot Principles
1. The efficiency of an irreversible heat engine is always less than the efficiency of a reversible one operating between same two thermal reservoirs.
2. The efficiencies of all reversible heat engines operating between the same two thermal reservoirs are the same.
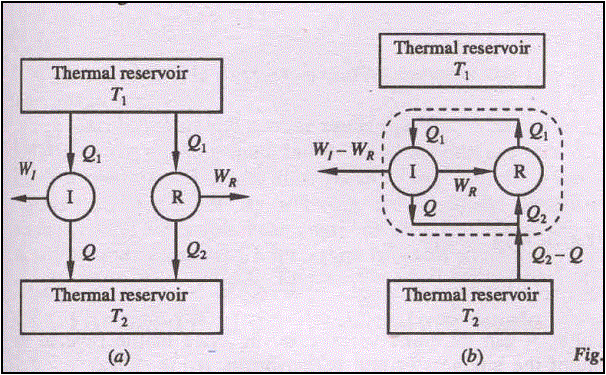
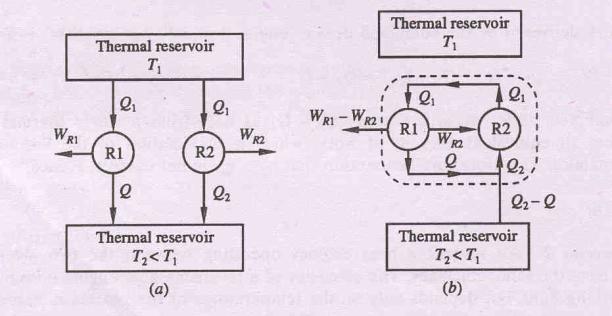
Lets us assume it is possible for an engine I to have an efficiency greater than the efficiency of a reversible heat engine R.
hI > hR
Let both the engines absorb same quantity of energy Q1. Let Q and Q2 represent the energy rejected as heat by the engines R, and I respectively.
WI = Q1 - Q
WR= Q1 – Q2
hI = WI / Q1 = (Q1 - Q)/Q1 = 1-Q/Q1
hR = WR/Q1 = (Q1 - Q2)/Q1 = 1-Q2/Q1
Since hI > hR,
1-Q/Q1 > 1-Q2/Q1
or, Q < Q2
Therefore, WI (=
Q1-Q) > WR (=Q1 – Q2)
Since the engine R is reversible, it can be
made to execute in the reverse order. Then, it will absorb energy Q2
from the reservoir at T2 and reject energy Q1 to the
reservoir at T1 when work WR is done on it.
If now engines I and R are combined, the net work delivered by the combined device is given by
WI – WR
= Q1 – Q – (Q1 – Q2) = Q2 – Q
The combined device absorbs
energy (Q2 – Q) as heat from a single thermal reservoir and delivers
an equivalent amount of work, which violates the second law of thermodynamics.
Hence, hR ³ hI
Carnot principle 2
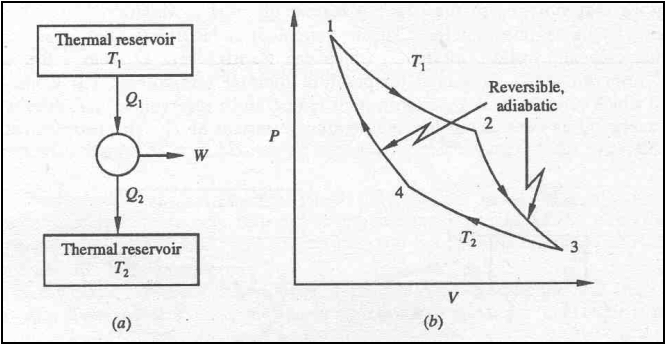
Consider two reversible heat
engines R1 and R2 , operating between the two given
thermal reservoirs at temperatures T1 and T2.
Let hR1 > hR2
Q1= energy
absorbed as heat from the reservoir at T1 by the engines R1 and R2,
separately.
Q = energy rejected by
reversible engine R1 to the reservoir at T2
Q2 = energy
rejected by reversible engine R2 to the reservoir at T2.
WR1 = Q1 - Q = work done by a reversible engine R1
.
WR2 = Q1
–Q2 = work done by a reversible engine R2
According to assumption,
hR1 > hR2
Or, 1 – Q/Q1 >
1- Q2/Q1
Q1 –Q >Q1-Q2
or WR1 >WR2
WR1 – WR2 = (Q1 –Q) – (Q1-
Q2) = Q2 – Q
Since the engine R2
is reversible, it can be made to execute the cycle in the reverse by supplying
WR2.
Since WR1 > WR2
the reversible engine R2 can be run as a heat pump by utilizing
part of the work delivered by R1.
For the combined device,
WR1 – WR2
= Q2 – Q, by absorbing energy Q2 – Q from a single
thermal reservoir which violates the second law of thermodynamics.
Hence hR1 > hR2 is incorrect.
By similar arguments, if we
assume that hR2 > hR1 then,
hR1 ³ hR2
Therefore, based on these
two equations,
hR1 = hR2
The efficiency of a
reversible heat engine is also independent of the working fluid and depends
only on the temperatures of the reservoirs between which it operates.