Entropy
1. 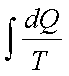 has the same value
irrespective of path as long as path is reversible
has the same value
irrespective of path as long as path is reversible
2. 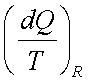 is an exact
differential of some function which is identical as entropy
is an exact
differential of some function which is identical as entropy
3. 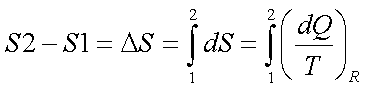
4. 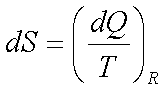 for reversible
process only
for reversible
process only
Calculation of Entropy change
1.
Entropy is a state function. The entropy change is determined by its
initial and final states only
2.
In analyzing irreversible process, it is not necessary to make a direct
analysis of actual reversible process.
Substitute actual
process by an imaginary reversible process. The entropy change for imaginary
reversible process is same as that of an irreversible process between given
final and initial states.
(a) Absorption of
energy by a constant temperature reservoir
Energy can be added reversibly or irreversibly as heat or by performing work.
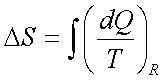
Example:-
The contents of a large constant-temperature reservoir maintained at 500 K are continuously stirred by a paddle wheel driven by an electric motor. Estimate the entropy change of the reservoir if the paddle wheel is operated for two hours by a 250W motor.
Paddle wheel work converted
into internal energy- an irreversible process. Imagine a reversible process with
identical energy addition
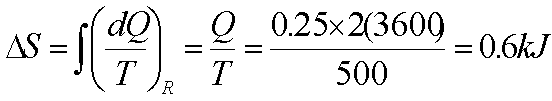
(b) Heating or cooling
of matter
![]() for constant volume
heating
for constant volume
heating
![]() for constant pressure
heating
for constant pressure
heating
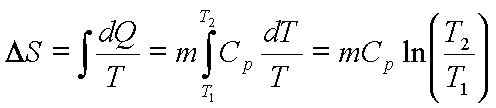
, for constant
pressure
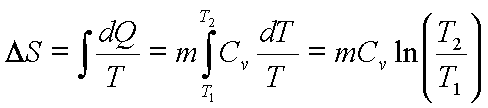
, for constant
volume process
Example: -
Calculate entropy
change if 1kg of water at 300 C is heated to 800C at 1
bar pressure. The specific heat of water is 4.2kJ/kg-K
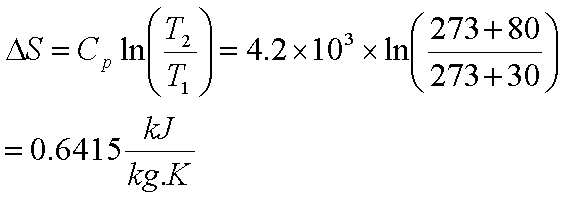
(c) Phase
change at constant temperature and pressure
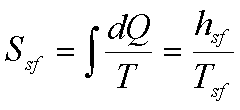
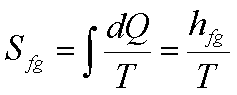
Example:-
Ice melts at 00C
with latent heat of fusion= 339.92 kJ/kg. Water boils at atmospheric pressure
at 1000C with hfg= 2257 kJ/kg.
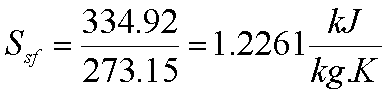
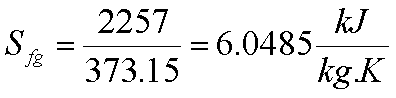
(d) Adiabatic
mixing
Example:-
A lump of steel
of mass 30kg at 4270 C is dropped in 100kg oil at 270C.The
specific heats of steel and oil is 0.5kJ/kg-K and 3.0 kJ/kg-K respectively.
Calculate entropy change of steel, oil and universe.
T= final
equilibrium temperature.
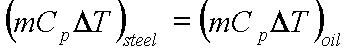
![]()
or T=319K
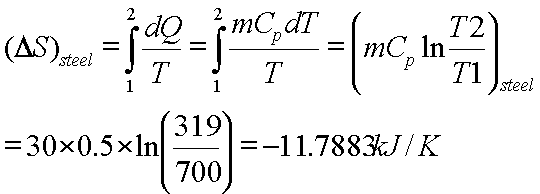
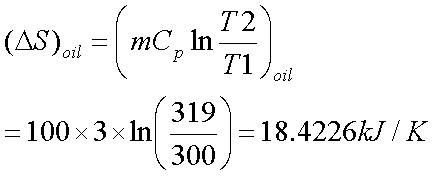
![]()
Tds relations
From the definition of entropy,
dQ = Tds
From the first law of
thermodynamics,
dW = PdV
Therefore,
TdS = dU + PdV
Or, Tds = du + Pdv
This is known as the first
Tds or, Gibbs equation.
The second Tds equation is obtained
by eliminating du from the above equation using the definition of enthalpy.
h = u + Pv à dh = du + vdP
Therefore, Tds = dh – vdP
The two equations can be
rearranged as
ds = (du/T) + (Pdv/T)
ds = (dh/T) – (vdP/T)
Change of state for an ideal gas
If an ideal gas undergoes a
change from P1, v1, T1 to P2, v2,
T2 the change in entropy can be calculated by devising a reversible
path connecting the two given states.
Let us consider two paths by
which a gas can be taken from the initial state, 1 to the final state, 2.
The gas in state 1 is heated
at constant pressure till the temperature T2 is attained and then it
is brought reversibly and isothermally to the final pressure P2.
Path 1-a: reversible,
constant-pressure process.
Path a-2: reversible,
isothermal path
Ds1-a = òdq/T = òCp dT/T = Cp
ln(T2/T1)
Dsa-2 = òdq/T = ò(du+Pdv)/T = ò(Pdv)/T = Rln(v2/va)
(Since du = 0 for an
isothermal process)
Since P2v2
= Pava = P1va
Or, v2/va = P1/P2
Or, Dsa-2 = -Rln(P2/P1)
Therefore, Ds = Ds1-a + Dsa-2
= Cp ln(T2/T1)
– Rln(P2/P1)
Path 1-b-2: The gas
initially in state 1 is heated at constant volume to the final temperature T2
and then it is reversibly and isothermally changed to the final pressure P2.
1-b: reversible, constant
volume process
b-2: reversible, isothermal
process
Ds1-b = Cv
ln(T2/T1)
Dsb-2 =Rln(v2/v1)
or, Ds = Cv
ln(T2/T1)+ Rln(v2/v1)
The above equation for Ds can also be deduced in the following manner:
ds = (dq/T)R = (du + Pdv)/T = (dh – vdP)/T
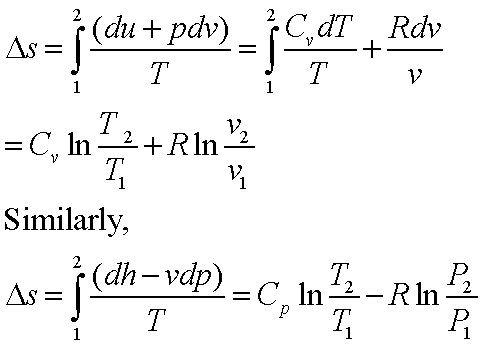
or,