Clapeyron
Equation
To find out the dependence
of pressure on equilibrium temperature when two phases coexist.
Along a phase transition
line, the pressure and temperature are not independent of each other, since the
system is univariant, that is, only one intensive parameter can be varied
independently.
When the system is in a
state of equilibrium, i.e., thermal, mechanical and chemical equilibrium, the
temperature of the two phases has to be identical, the pressure of the two
phases has to be equal and the chemical potential also should be the same in
both the phases.
Representing in terms of
Gibbs free energy, the criterion of equilibrium is:
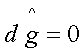 at constant T and P
at constant T and P
or, 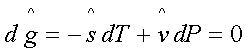
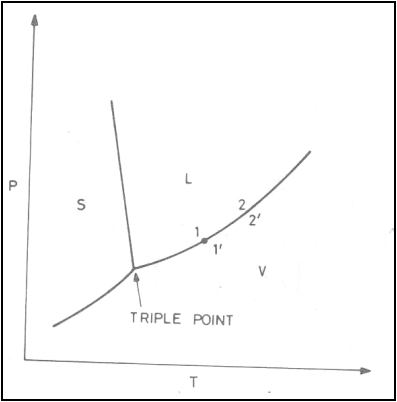
Consider a system consisting
of a liquid phase at state 1 and a vapour phase at state 1’ in a state of
equilibrium. Let the temperature of the system is changed from T1 to
T2 along the vaporization curve.
For the phase transition for
1 to 1’:
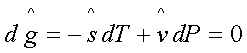
or 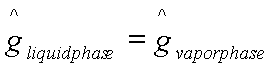
or 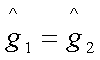
In reaching state 2 from
state 1, the change in the Gibbs free energy of the liquid phase is given by:
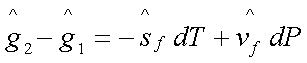
Similarly, the change in the
Gibbs free energy of the vapour phase in reaching the state 2’ from state 1’ is
given by:
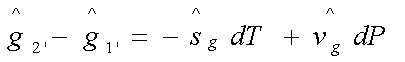
Therefore, 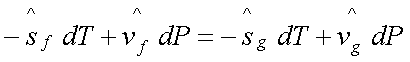
Or 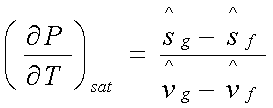
Where the subscript sat implies
that the derivative is along the saturation curve.
The entropy change
associated with the phase transition:
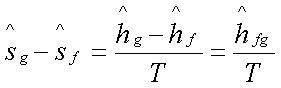
Hence, 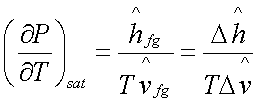
Which is known as the Clapeyron
equation
Since ![]() is always positive
during the phase transition,
is always positive
during the phase transition, 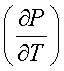 sat will be positive or negative depending upon
whether the transition is accompanied by expansion (
sat will be positive or negative depending upon
whether the transition is accompanied by expansion (![]() >0) or contraction (
>0) or contraction (![]() <0).
<0).
Consider the liquid-vapour
phase transition at low pressures. The vapour phase may be approximated as an
ideal gas. The volume of the liquid phase is negligible compared to the volume
of the vapour phase(![]() >>
>> ![]() )and hence
)and hence ![]() =
= ![]() =
=![]() =RT/P.
=RT/P.
The Clapeyron equation becomes:
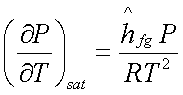
or 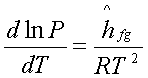
which is known as the Clausius-Clapeyron
equation.
Assume that ![]() is constant over a
small temperature range, the above equation can be integrated to get,
is constant over a
small temperature range, the above equation can be integrated to get,
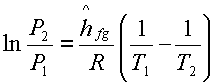
or 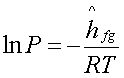 +constant
+constant
Hence, a plot of lnP
versus 1/T yields a straight line the slope of which is equal to –(hfg/R).
Kirchoff Equation
Kirchoff relation predicts
the effect of temperature on the latent heat of phase transition.
Consider the vaporization of
a liquid at constant temperature and pressure as shown in figure. The latent
heat of vaporization associated with the phase change 1 to 1’ is (![]() -
-![]() ) at temperature T. When the saturation temperature is raised
to (T+dT), the latent heat of vaporization is (
) at temperature T. When the saturation temperature is raised
to (T+dT), the latent heat of vaporization is (![]() -
-![]() ). The change in latent heat,
). The change in latent heat,
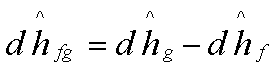
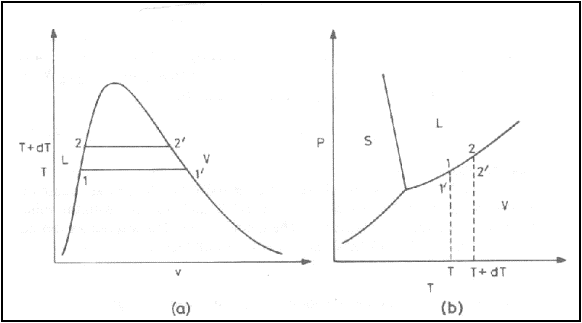
The variation in the
enthalpy associated with the variation in the independent variables T and P is
given by:
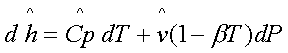
or,
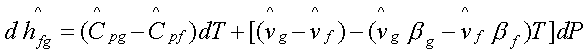
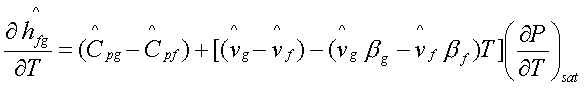
Substituting for (dP/dT)sat
from the clapeyron equation,
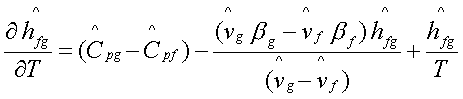
This is known as Kirchoff
relation.
For a solid-to-liquid transition, it is a reasonably good approximation to assume that the molar heat capacity and the molar volume are constant in each phase and the coefficient of volume expansion b is negligible for each phase. Then,
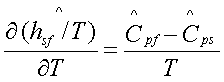
where ![]() is the latent heat of
fusion.
is the latent heat of
fusion.
For the transition from
liquid phase to vapour phase, the molar volume of the liquid phase can be
neglected compared to the molar volume of the gas phase, and bg>>bf. The vapour
phase may be approximated as an ideal gas. Then bg=1/T. It is clear
that vgbg> vfbf. Hence,
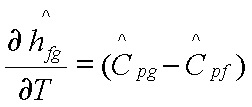
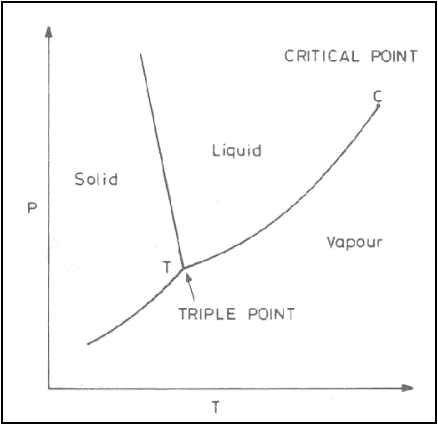
Phase Equilibrium- Gibbs Phase Rule
The number of independent
variables associated with a multi component, multiphase system is given by the Gibbs
Phase Rule, expressed as,
F=C+2-P
Where,
F= The number of independent
variables
C= The number of components
P= The number of phases
present in the equilibrium
· For a single
component (C=1) two phase (P=2) system, one independent intensive property needs
to be specified (F=1).
· At the triple
point, for C=1, P=3 and thus F=0. None of the properties of a pure substance at
the triple point can be varied.
· Two independent
intensive properties need to be specified to fix the equilibrium state of a
pure substance in a single phase.
Phase diagram for a single
component system is given in figure.