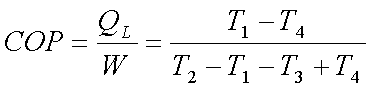Carnot Vapour compression Refrigeration cycle

(a) Schematic representation
(b) T-s diagram
Processes: -
1-2: Isentropic compression from state 1 (wet vapour) to state 2 (saturated vapour)
2-3: Heat rejection (QH)
in the condenser
3-4: Isentropic expansion
from state 3 (saturated liquid)
4-1: Heat absorption ( QL)
in the evaporator
The COP of the refrigerator,
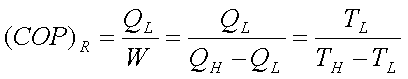
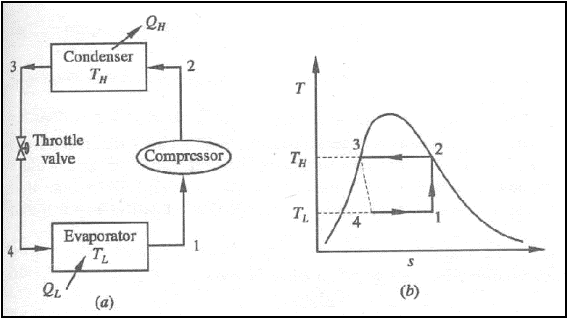 Practical Vapour compression refrigeration cycle
Practical Vapour compression refrigeration cycle
(a) schematic diagram (b)
T-s diagram
Application of the first law
of thermodynamics to the control volume compressor, condenser, throttle and
evaporator gives
(Ws)compressor=h2-h1
QH=h2-h3
h3=h4
and QL=h1-h4
The COP of the refrigerator
is given by,
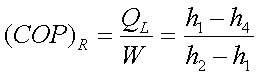
In the ideal refrigeration
cycle, the refrigerant leaves the evaporator as wet vapour.
In some cases the
refrigerant leaves the evaporator as either saturated vapour or superheated
vapour.
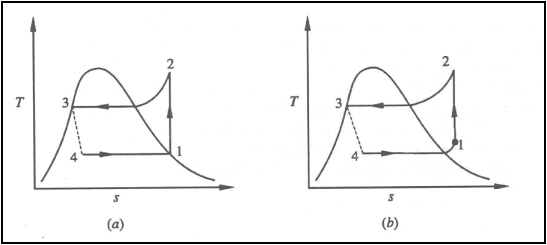
T-s diagram for a vapour
compression refrigeration cycle when the refrigerant leaves the evaporator as
(a) saturated vapour (b) superheated vapour
Gas refrigeration cycle
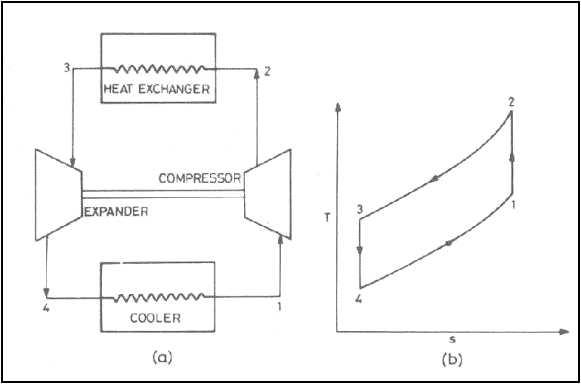
(a) Schematic diagram (b)
T-s diagram
The simplest gas
refrigeration cycle is the reversed Brayton cycle
Processes: -
1-2: isentropic compression
for state 1 (atmospheric air) to state 2
2-3: energy exchange with
the surrounding, air is cooled
3-4: isentropic expansion to
state 4
Work obtained during the expansion process can be used to run the
compressor
Work done on the compressor,
![]()
Work delivered by the
expander,
![]()
The net work required= CP
(T2-T1-T3+T4)
The COP of this
refrigeration system is given by,