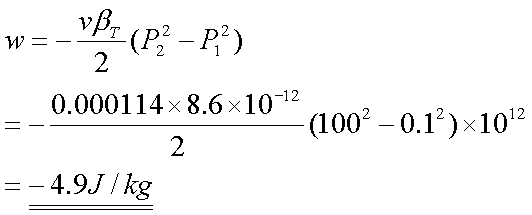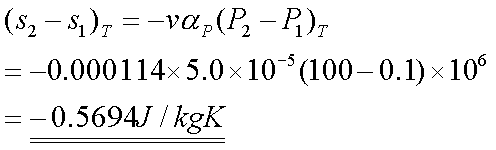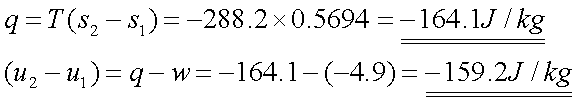Problems
Problem 1:-
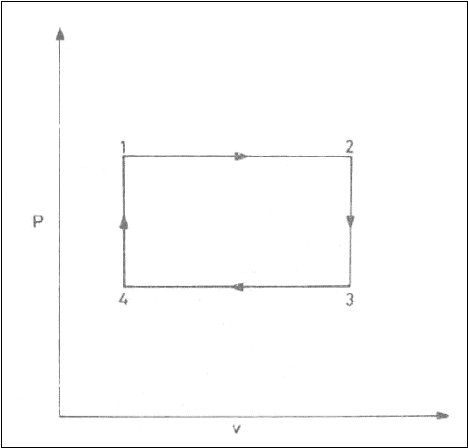
Evaluate the slopes of a
constant-pressure process and a constant- volume process lines on an enthalpy versus
pressure diagram and on an enthalpy versus entropy diagram,
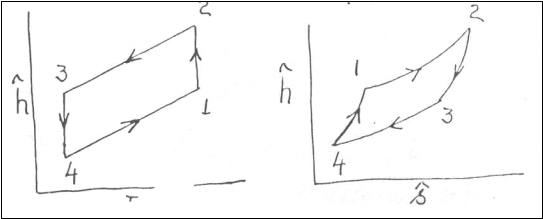
respectively, for an ideal gas. Redraw the cycle shown below on h-P and h-s
diagrams?
Solution 1:-
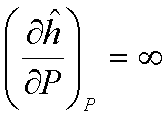
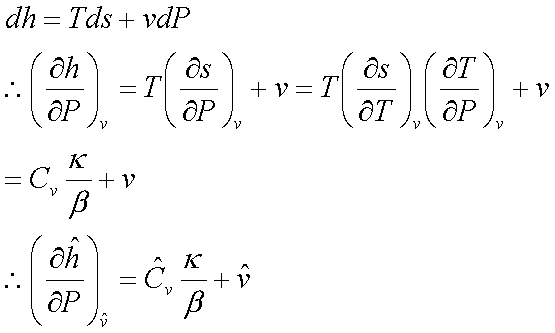
For an ideal gas: 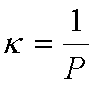 and
and 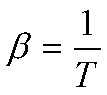
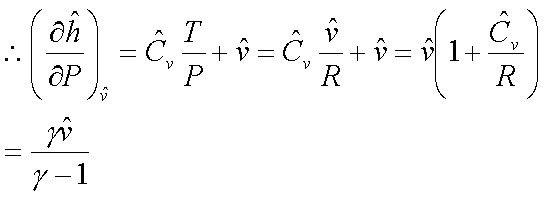
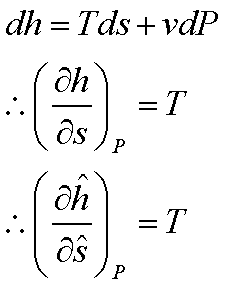
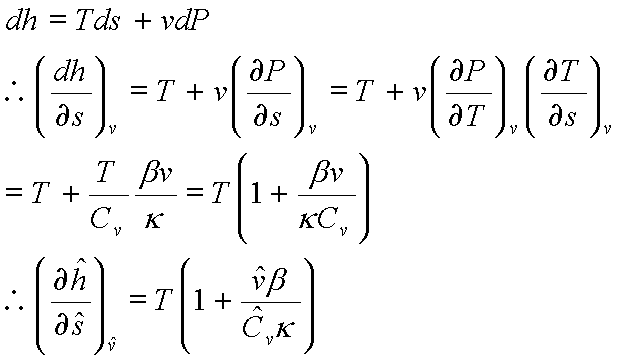
For an ideal gas:
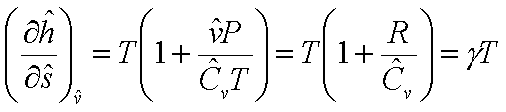
Problem 2:-
The decrease in the melting
point of ice with increase in pressure is largely responsible for our ability
to skate on ice. At the line of contact between the skate and the ice a high
pressure is exerted by the person who is skating because of the small area of
contact. Therefore, ice melts and provides a thin layer of liquid water which
acts as a lubricant and help in skating. Suppose a man of mass 75 kg desires to
skate on ice. The area of contact between the skates and ice is 20 mm2.
Will he able to skate on ice, which is at –20 C?
Solution 2:-
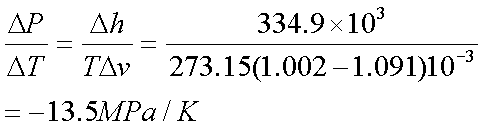
If DT=-2K, pressure required =
27MPa
Pressure increase due to the
person on skates
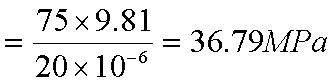
Since the increase in
pressure is greater than the required pressure, it is possible to skate.
Problem 3:-
Determine the sublimation
pressure of water vapour at –600C using data available in the steam
tables.
Control
mass: water
From steam tables,
For saturated solid-
saturated vapour water,
|
Temp.,T C |
Pressure, P kPa |
Evap.Enthalpy, hig KJ/kg |
|
-30 |
0.03810 |
2839.0 |
|
-40 |
0.01286 |
2838.9 |
Solution 3:-
For sublimation, the change
from solid ‘i’ directly to vapour ‘g’, the Clapeyron equation reduces to the
form,
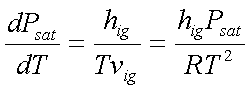
hig is relatively
constant in these temperature ranges. Hence, integrate between the limits –400C
and –600C.
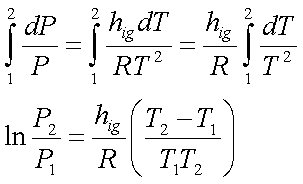
Let
P2 =
0.0129kPa T2=233.2K T1=213.2K
Then,
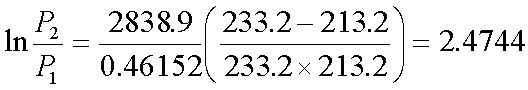
P1
= 0.00109kPa
Problem 4:-
The pressure on a block of
copper having a mass of 1kg is increased in a reversible process from 0.1 to
100 MPa while the temperature is held constant at 150C. Determine
the work done on the copper during this process, the change in entropy per kilogram
of copper, the heat transfer, and the change of internal energy per kilogram.
Over the range of pressure
and temperature in this problem, the following data can be used:
Volume expansivity = aP =5.0*10-5K-1
Isothermal compressibility=bT=8.6*10-12m2/N
Specific volume= 0.000114 m3/kg
Analysis:-
Control mass: copper block
States: initial and final
states known
Process: Constant
temperature, reversible
The work done during the
isothermal compression is
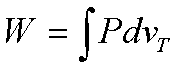
The isothermal
compressibility has been defined as
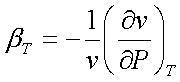
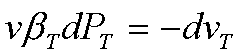
Therefore, for this
isothermal process,
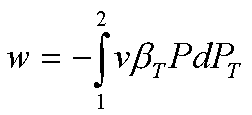
Since v and bT remain
essentially constant, this is readily integrated:
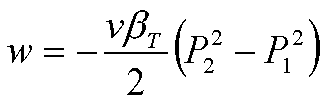
The change of entropy can be
found by considering the Maxwell relation, and the definition of volume
expansivity.
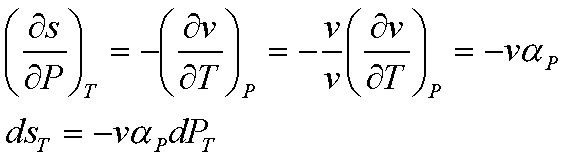
This equation can be readily
integrated, if we assume that v and aP remain constant:
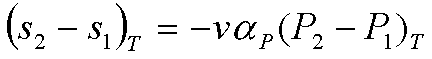
The heat transfer for this
reversible isothermal process is
q =
T(s2-s1)
The change in internal
energy follows directly from the first law,
(u2-u1)=
q-w
Solution 4:-