Energy flow in hydrogen-bonded microclusters
Multiple hydrogen bonds are thought to
play important role in facilitating photo-induced charge and electronic energy
transfer processes, particularly in Biology.
For unambiguous demonstrations of the effects of multiple hydrogen bonds
in chemical dynamics, an important requirement is to work on clean
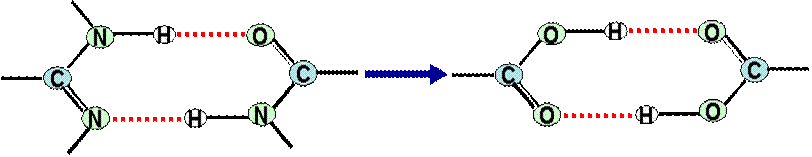
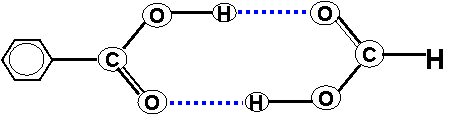
systems. We have been using the dimers of carboxylic acids as models of many of the doubly
hydrogen bonded natural systems as shown here.
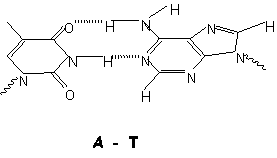
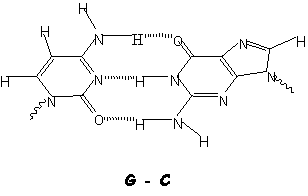
Doubly H-bonded DNA base pair (top) and
structural model (below)
A comparison of the binding energies of selected H-bonded dimmers are presented
here.


The data shows that energetically the
H-bonds in carboxylic acid dimers are almost
equivalent to the well-characterized dimers between
two molecules of water, phenol and hydrogen fluoride. However, the unusual characteristics of the
doubly H-bonded dimers are revealed in both the IR
and electronic spectra. A few examples
of our recently measured systems are presented.
![]()
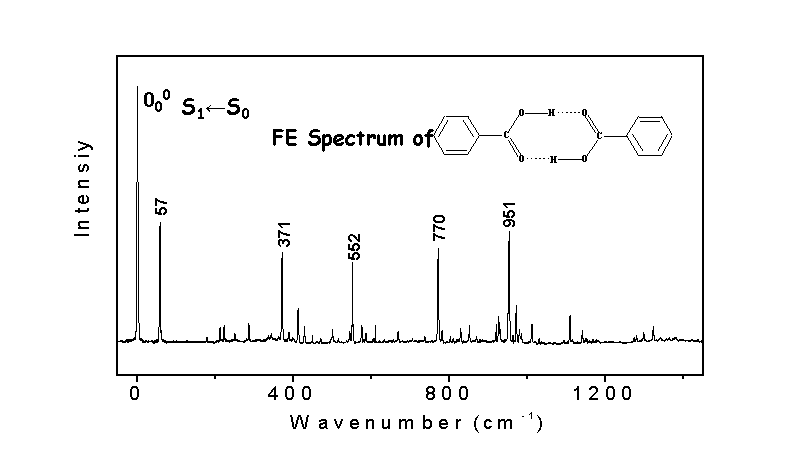
The spectrum shown here is the
fluorescence excitation spectrum of benzoic acid dimer. The most unusual feature is the appearance of
an intense low-frequency band at 57 cm-1 above the S1←S0
origin band. However, no progression
corresponding to this transition is visible in the spectrum.
Nandi and Chakraborty
JCP, 120, 8521 (2004)
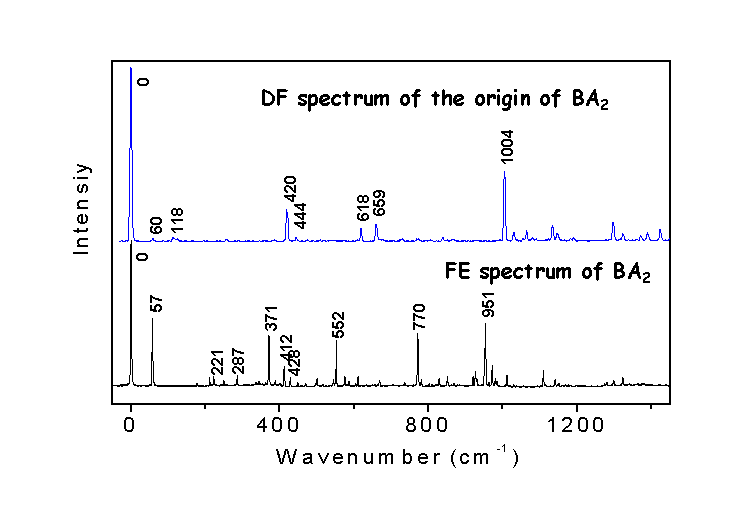
A comparison between the fluorescence
excitation spectrum and dispersed fluorescence spectrum from the S1
origin of benzoic acid dimer is shown here.
It shows a loss of mirror symmetry relation between the two spectra,
particularly region between the two spectra.
Nandi and Chakraborty
JCP, 120, 8521 (2004)
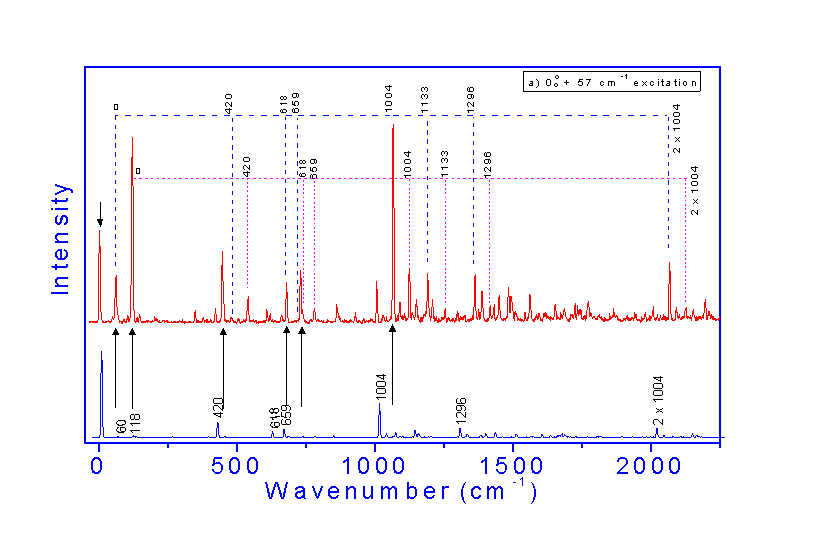
The comparison between the DF spectrum of
the 57 cm-1 band with that of the electronicorigin
shows that the former originates due to extensive vibrational mixing of the intramolecular
modes with the doubly H-bonded intermolecular
modes of the dimer in S1.
Nandi and Chakraborty
JCP, 120, 8521 (2004)
Hydrogen bond mediated rotor-ring coupling in acetic acid
benzoic acid mixed dimer
It is believed over a long time that
methyl rotor plays vital role to modulate the dynamics of electronically
excited states. We show here that doubly H-bonded interface behaves as an
efficient conduit to transmit the rotor effect to promote IVR of the phenyl
ring. A comparison of fluorescence excitation and dispersed fluorescence
spectra of following two mixed dimers are presented.

Fluorescence excitation
spectra of the two mixed dimers. The vibronic structures in S1 of both species are
identical. The bands labeled
by asterisks are the homodimers of BA
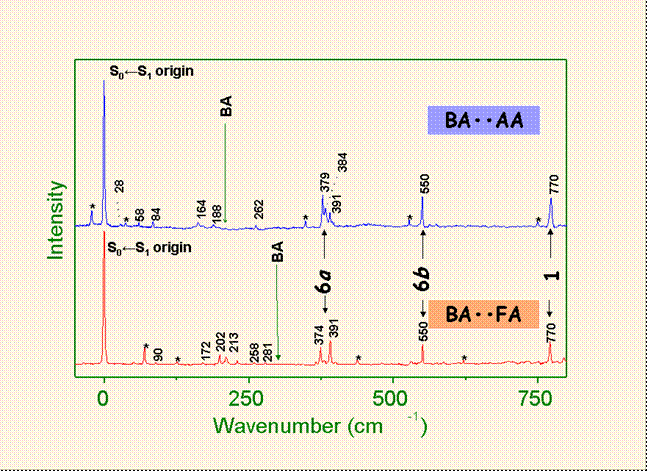
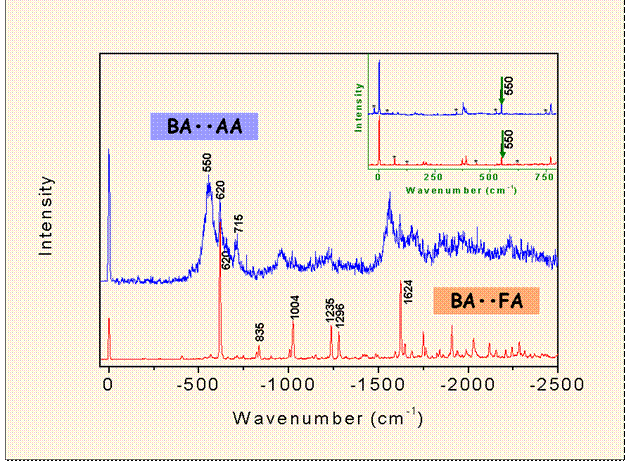
The comparison between the DF spectra for
6b01 excitations of two dimers
shows that methyl group induced extensive IVR in the Acetic acid-Benzoic acid
mixed dimer, whereas the spectrum of the formic acid-benzoic acid dimer
exhibits quite regular behavior.