Throttling Process:
The porous plug experiment was designed to measure temperature changes when a fluid flows steadily through a porous plug which is inserted in a thermally insulated, horizontal pipe. The apparatus used by Joule and Thomson is shown in Figure 14.1.
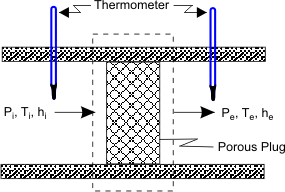
Figure 14.1
A gas at pressure 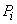 and temperature and temperature 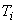 flows continuously through a porous plug in a tube and emerges into a space which is maintained at a constant pressure flows continuously through a porous plug in a tube and emerges into a space which is maintained at a constant pressure 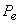 . The device is thermally insulated and kept horizontal. Consider the dotted portion as control volume. . The device is thermally insulated and kept horizontal. Consider the dotted portion as control volume. 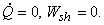 This results in This results in 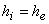
Therefore, whenever a fluid expands from a region of high pressure to a region of low pressure through a porous plug, partially opened valve or some obstruction, without exchanging any energy as heat and work with the surrounding (neglecting, the changes in PE and KE), the enthalpy of the fluid remains constant, and the fluid is said to have undergone a throttling process. If the downstream pressure is held at several different values successively and at each of these 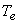 is measured, we shall obtain a situation explained by Figure 14.2. is measured, we shall obtain a situation explained by Figure 14.2.
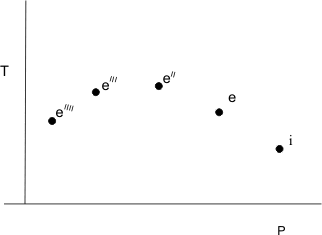
Figure 14.2
Each plotted point represents a state for which the enthalpy is equal to 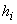 . If we join all these points we shall be able to obtain a constant enthalpy line (figure 14.3). . If we join all these points we shall be able to obtain a constant enthalpy line (figure 14.3). 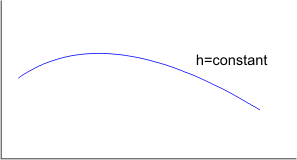
Figure 14.3 Such a plot of P versus 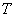 yields may also be called isenthalpic curve. The slope of the isenthalpic curve is called the Joule-Thomson coefficient and given by yields may also be called isenthalpic curve. The slope of the isenthalpic curve is called the Joule-Thomson coefficient and given by
The experiments are conducted with different 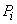 and and 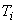 in order to find out the isenthalpic curves. A family of isenthalpic curves is shown in Figure 14.4 which is typical of all real gases. in order to find out the isenthalpic curves. A family of isenthalpic curves is shown in Figure 14.4 which is typical of all real gases.
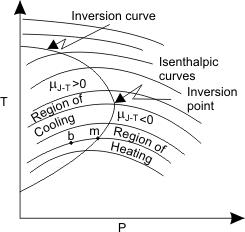
The point at which 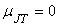 called the inversion point. The locus of all in version points is the inversion curve. called the inversion point. The locus of all in version points is the inversion curve.
In the region left of the inversion curve, 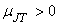 . In the throttling process the down stream pressure . In the throttling process the down stream pressure 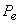 is always less than the upstream pressure is always less than the upstream pressure 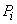 . Therefore, whenever a real gas is subjected to throttling, the temperature of the gas decreases if the initial state lies in the region to the left of the isenthalpic curve. This is explained by a process from . Therefore, whenever a real gas is subjected to throttling, the temperature of the gas decreases if the initial state lies in the region to the left of the isenthalpic curve. This is explained by a process from 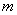 to to 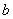 in Figure 14.4. in Figure 14.4.
To the right of the inversion curve, 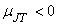 . If the initial state of gas lies in the region to right of the inversion curve, the temperature of the gas increase upon throttling. For almost all the gases, at ordinary range of pressures and temperature, . If the initial state of gas lies in the region to right of the inversion curve, the temperature of the gas increase upon throttling. For almost all the gases, at ordinary range of pressures and temperature, 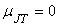 and the maximum inversion temperature is above the room temperature. The exceptions are hydrogen, helium and neon. For hydrogen, the maximum inversion temperature is 200 K and for helium the maximum inversion temperature is 24 K. If hydrogen is throttled at room temperature, the temperature of the gas increases. To produce low temperature by throttling, the initial temperature of hydrogen should be below 200 K. This is usually accomplished by cooling with liquid nitrogen. Similarly, in the production of liquid helium by throttling, the initial temperature of helium should be below 24 K. Hence it is cooled by liquid hydrogen prior to throttling. and the maximum inversion temperature is above the room temperature. The exceptions are hydrogen, helium and neon. For hydrogen, the maximum inversion temperature is 200 K and for helium the maximum inversion temperature is 24 K. If hydrogen is throttled at room temperature, the temperature of the gas increases. To produce low temperature by throttling, the initial temperature of hydrogen should be below 200 K. This is usually accomplished by cooling with liquid nitrogen. Similarly, in the production of liquid helium by throttling, the initial temperature of helium should be below 24 K. Hence it is cooled by liquid hydrogen prior to throttling.
Suppose an ideal gas is throttled. Since throttling process is isenthalpic, and for an ideal gas enthalpy is a function of temperature only, the temperature of an ideal gas does not change during a throttling process. Hence 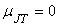 for an ideal gas. for an ideal gas.
|