Clausius Statement of the Second Law
Heat always flows from a body at higher temperature to a body at a lower temperature. The reverse process never occurs spontaneously. Clausius' statement of the second law gives: It is impossible to construct a device which, operating in a cycle, will produce no effect other than the transfer of heat from a low-temperature body to a high temperature body.
This statement tells us that it is impossible for any device, unaided by an external agency, to transfer energy as heat from a cooler body to a hotter body. Consider the case of a refrigerator or a heat pump (Figure 17.2)
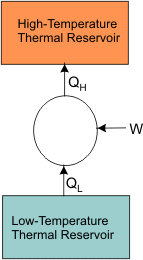
Figure 17.2 When 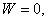
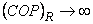 and and
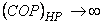
It is impossible to construct a refrigerator or a heat pump whose COP is infinity. Consider a domestic refrigerator, this device extracts energy as heat from the substance to be coded and transfers it to the surroundings. The refrigerator is supplied with electric power. Energy transfer as heat from a high temperature body to a low temperature body is a spontaneous process. The Clausius statement of the second law of thermodynamics tells that this spontaneous process cannot proceed in the reverse direction.
Apparently, the Kelvin Planck statement and the Clausius statement of the second law of thermodynamics are altogether different. They are not ! Instead, they are equivalent. A violation of Kelvin Planck statement leads to a violation of the Clausis statement too and vice-versa.
|