Reversible Isothermal Heat Addition
In the first process, the cylinder head is brought into contact with a source at temperature 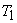 . The gas inside the cylinder is also at temperature . The gas inside the cylinder is also at temperature 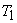 . The gas expands reversibly and isothermally . During this process, the system absorbs energy as heat . The gas expands reversibly and isothermally . During this process, the system absorbs energy as heat  from the source. The system changes its state from 1 to 2 on the from the source. The system changes its state from 1 to 2 on the  diagram. diagram.
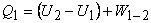 |
(18.1) |
where, for an ideal gas, 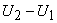
Reversible Adiabatic Expansion
In the second process, the cylinder head is insulated and the gas is allowed to expand till its temperature is equal to the sink temperature 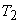 . The system thus reaches state 3. This is a reversible adiabatic process. . The system thus reaches state 3. This is a reversible adiabatic process.
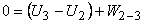 |
(18.2) |
Reversible Isothermal Heat Rejection
In the next process, the system is brought into contact with the sink which is at a temperature 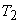 . The heat . The heat 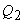 leaves the system and the internal energy further decreases leaves the system and the internal energy further decreases
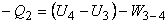 |
(18.3) |
where, only for an ideal gas, 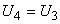
Through a reversible isothermal process the system reaches state 4.
|