Intensive and Extensive Properties
There are certain properties which depend on the size or extent of the system, and there are certain properties which are independent of the size or extent of the system. The properties like volume, which depend on the size of the system are called extensive properties. The properties, like temperature and pressure which are independent of the mass of the system are called intensive properties. The test for an intensive property is to observe how it is affected when a given system is combined with some fraction of exact replica of itself to create a new system differing only by size. Intensive properties are those which are unchanged by this process, whereas those properties whose values are increased or decreased in proportion to the enlargement or reduction of the system are called extensive properties.
Assume two identical systems S1 and S2 as shown in Figure 2.1 . Both the systems are in identical states.
Let S3 be the combined system. Is the value of property for S3 same as that for S1 (and S2 )?
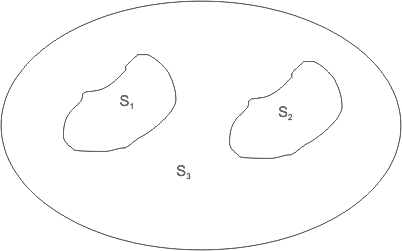
Figure 2.1
- If the answer is yes, then the property is intensive
- If the answer is no, then the property is extensive
The ratio of the extensive property to the mass is called the specific value of that property
specific volume, v = V/m = 1/ ρ ( ρ is the density)
specific internal energy, u = U/m
Similarly, the molar properties are defined as the ratios of the properties to the mole number (N) of the substance
Molar volume = 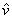 = V/N = V/N
Molar internal energy =  = U/N = U/N
|