Entropy change of an incompressible substance
- For most liquids and all solids the density is not changed as pressure changes, that is,
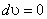 . Gibbsian equation states that, . Gibbsian equation states that, 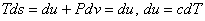 , for an incompressible substance , for an incompressible substance 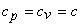 is a function of temperature only. is a function of temperature only.
Therefore, 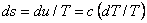
Integrate to determine the entropy change during a process
where 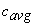 is the averaged specific heat of the substance over the given temperature range. is the averaged specific heat of the substance over the given temperature range.
- Specific heat for some common liquids and solids can be found in thermodynamic tables.
Example Problem 2
-
An 1-kg metal bar initially at 1000 K is removed from an oven and quenched by immersing in a closed tank containing 20 kg of water initially at 300 K. Assume both substance are incompressible and c (water) = 4 (kJ/kg K), c (metal) = 0.4 (kJ/kg K). Neglect heat transfer between the tank and its surroundings. (a) Determine the final temperature of the metal bar, (b) entropy generation during the process.
Solution
- Energy balance from the first law:
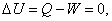 no heat transfer and no work done no heat transfer and no work done
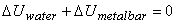 , both bar and water reach final temperature , both bar and water reach final temperature 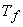 . .
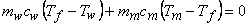
- No heat transfer with surroundings
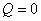 . The entropy balance of the system . The entropy balance of the system 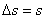 (generation) = (generation) = 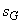
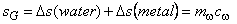 In In 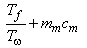 In In 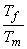
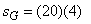 In In 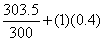 In In 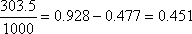 ( kJ / K ) ( kJ / K )
The total entropy of the system increases, thus satisfies the second law.
Example Problem 3
Steam enters an adiabatic turbine at 5 MPa and 4500C and leaves at a pressure of 1.4 MPa. Determine the work output of the turbine per unit mass flowing through the turbine if we can assume the process is reversible and neglect all changes of KE and PE. Refer to Figure 24.3
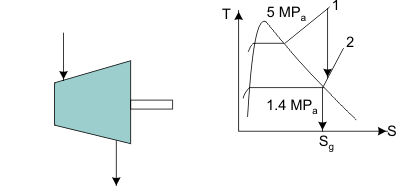
Figure 24.3 
State 1: 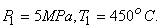 F From table: F From table: 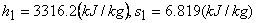 State 2: 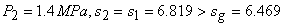 at 1.4M Pa (from table) at 1.4M Pa (from table)
State 2 is superheated. From table,
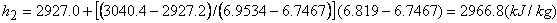
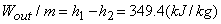
|