The highest possible efficiency in a power cycle can be obtained if the cycle consists of only reversible processes. Therefore, a Carnot cycle is quite appealing as a power cycle. Consider the Carnot cycle shown in Figure 27.1
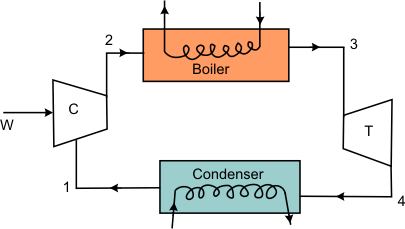
Figure 27.1
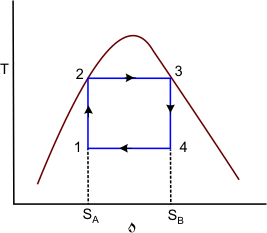
Figure 27.2
Figure 27.2 is the T-s diagram of the Carnot cycle shown in Figure 27.1. Wet steam at state 1 is isentropically compressed to the saturated liquid (state 2). The saturated liquid undergoes a phase change of constant temperature and pressure and leaves the boiler as saturated vapour (state 3). Then the saturated vapour is allowed to expend isentropically in a turbine and the leaves the turbine at state 4. Finally this fluid is condensed to state 1, thus completing the cycle. Energy is added to the working fluid at constant temperature 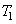 and energy is rejected at constant temperature and energy is rejected at constant temperature 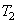 in the condensed. in the condensed.
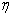 = = 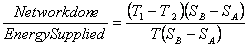 |
(27.1) |
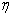 = 1- = 1-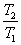 |
(27.2) |
The isentropic process 1-2 cannot be practically achieved become it is difficult to handle a two phase mixture. Similarly if the quality is poor, the process 3-4 is also difficult to carry out.
The ideal Rankine Cycle shown in Figure 27.3 is a modification of Carnot cycle.
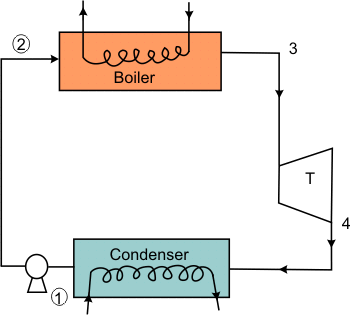
Figure 27.3
The isentropic compression of wet steam is replaced by isentropic compression of saturated liquid which can be easily carried out with the help of a pump. Figure 27.4 is the cyclic representation of the modified Carnot cycle.
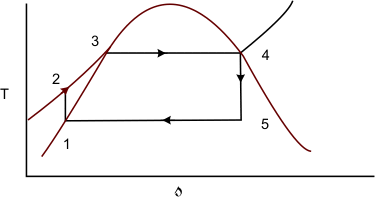
Figure 27.4
Energy addition
Energy rejection
Thermal efficiency 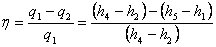 |
(27.3) |
or
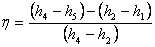 = =  |
(27.4) |
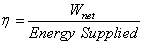 |
(27.5) |
It can be observed that the isentropic expansion of the steam continuously decreases its quality when going from state 4 to state 5. Presence of excessive moisture content causes serious erosion of the turbine blades, which is highly undesirable. To overcome this modern steam power plants produce superheated steam which is fed to the turbine for subsequent expansion (Figure 27.5)
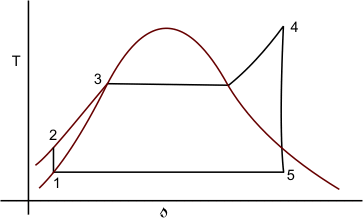
Figure 27.5
The thermal efficiency of a Rankine cycle is lower then that of a Carnot cycle operating between the same temperature levels. This is primarily because of the fact that the energy transfer as heat in the boiler does not take place at constant temperature in the Rankine cycle. The average temperature between the state 2 and 3 is less than the temperature at which vaporization take place. Particularly in the case of Rankine cycle with superheat, the maximum temperature corresponds to state 4 which is much above the temperature of vaporization at which a major fraction of the energy addition take place.
|