Introduction
In thermodynamics, an important task is to develop relationships which express the nonmeasurable properties like U,H,A,G and S in terms of measurable properties P,V & T The number of partial derivatives of the type 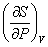 among the above eight properties are among the above eight properties are 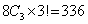
If there exists a relation S=S (T,P) among the three variables S,T,P then
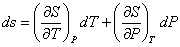 |
(32.1) |
Dividing the above relation with dT while V is held constant, we obtain
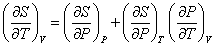 |
(32.2) |
There exists one relationship among four partial derivatives
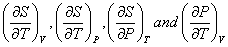 |
(32.3) |
Therefore a large number of relationships is possible among 336 partial derivatives.
|