Clapeyron Equations
Let us study v−T diagram of a system. The system consists of a liquid phase at state A and a vapour phase at state B (figure 33.1)
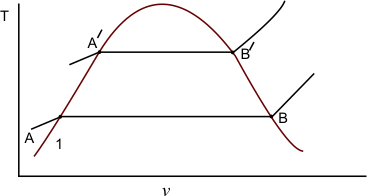
The line AB corresponds to the pressure P and temperature T. Gibbs free energy can be conveniently used in analyzing the problem. Using Gibbs equation we can write
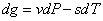 |
(33.1) |
Along AB both dP and dT vanish; therefore dg=0
Hence, Gibbs function at a given pressure and temp has the same value for the saturated liquid and the saturated vapour.
The same result holds good for solid-liquid transition.
Next, consider a neighbouring line 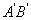 corresponding to pressure corresponding to pressure 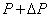 and temperature and temperature 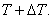 The value of the specific Gibbs function on the line The value of the specific Gibbs function on the line 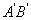 may be denoted by may be denoted by 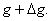 Then assuming Then assuming 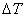 etc to be small, the change along etc to be small, the change along 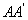 is given by is given by
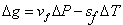 |
(33.2) |
While the same along 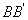 is given by is given by
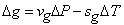 |
(33.3) |
Hence form (33.2) and (33.3) we get,
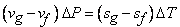 |
(33.5) |
Diving by 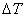 and proceeding to the limit and proceeding to the limit 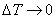
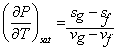 |
(33.5) |
As transition from A to B is a constant pressure and constant temperature process, we get
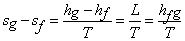 |
(33.6) |
Where L or 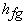 is the latent heat of evaporation at temperature T is the latent heat of evaporation at temperature T
Hence
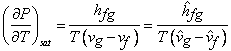 |
(33.7) |
This is known as Clapeyron Equation. Also valid for solid - liquid transition.
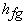 is always positive so is always positive so 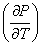 will depend on will depend on 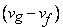 - Liquid
 vapour: vapour:  is always positive. is always positive.
At higher pressure, boiling point is higher.
- But for solid
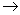 liquid: sometimes liquid: sometimes 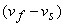 is negative; Notably for water: Melting is accompanied by contraction. A rise is pressure will lower down the melting point. (figure 33.2) This explains the phenomenon of relegation. is negative; Notably for water: Melting is accompanied by contraction. A rise is pressure will lower down the melting point. (figure 33.2) This explains the phenomenon of relegation.
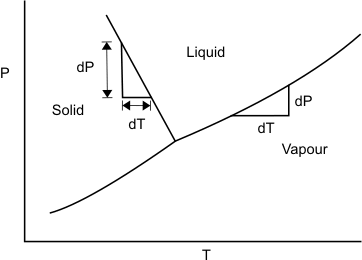
The Clapeyron equation can be put in a simple form if we make certain approximations. Let us consider the liquid – vapour phase transition at low pressures. Then the vapour phase may be approximated as an ideal gas. Moreover the volume if the liquid phase is negligible compared to the volume of the vapour phase 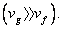
Hence
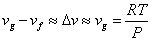 |
(33.8) |
Using these condition, Clapeyron equation becomes
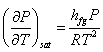 |
(33.9) |
or
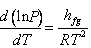 |
(33.10) |
Which is known as Clausius – Clapeyron equation further, if we assume that 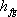 is constant over a small temperature range, the above equation may be integrated to get is constant over a small temperature range, the above equation may be integrated to get
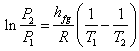 |
(33.11) |
or
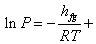 Constant Constant |
(33.12) |
Therefore a plot of InP versus 1/T yields a straight line the slope of which is equal to 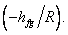
The vapour pressure of most the substances agree fairly well with this equation.
|