TWO SPECIAL CASES:
- In solid to liquid transition, the specific volume and specific heat are constant in each phase and the value of
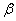 in each phase is negligible; consequently, in each phase is negligible; consequently,
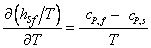 |
(33.21) |
Where hsf is the latent heat of Fusion
- If the transition is from liquid to ideal gas, then
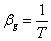
Also, 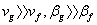 so that so that 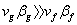
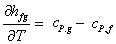 |
(33.22) |
This equation is also applicable in the case of sublimation with subscript f (for liquid) replaced by subscript s (for solid).
Phase Equilibrium
If two phases are in a state of equilibrium, the temperature and pressure of both the phases must be identical. The chemical potential 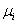 of component of component 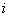 must be identical in both the phases. That is must be identical in both the phases. That is
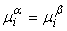 for all for all 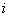 |
(33.23) |
Where 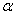 and and 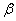 refer to two different phases. refer to two different phases.
The criterion of equilibrium at constant temperature and pressure is given by 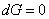
|