Properties of Atmospheric Air
Dry air is a mechanical mixture of the following gases: Oxygen, nitrogen, carbon dioxide, hydrogen, argon, neon, krypton, helium, ozone, and xenon. Dry air is considered to consist of 21% oxygen and 79% nitrogen by volume. It consists of 23% oxygen, and 77% nitrogen by mass. Completely dry air does not exist in nature. Water vapour in varying amount is diffused through it. If Pa and Pw are the partial pressures of dry air and water vapour respectively, then by Dalton's law of partial pressure
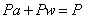 |
(35.1) |
Where P is the atmospheric pressure
Mole – fraction of dry air,
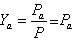 |
(35.1) |
Pm is considered to be 1 atm
Mole – fraction of water vapour,
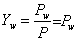 |
(35.3) |
Since 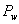 is very small, the saturation temperature of water vapour at is very small, the saturation temperature of water vapour at 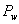 is less than the atmospheric temperature is less than the atmospheric temperature 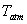 . So the water vapour in air exists in the superheated state, and the air is said to be unsaturated. . So the water vapour in air exists in the superheated state, and the air is said to be unsaturated.
If the air- water vapour mixture which is initially not saturated, is cooled at constant pressure, the partial pressure of water vapour in the mixture remains constant till it is equal to the saturation pressure of water. Further cooling result in condensation of water vapour. The temperature at which the vapour condenses when the air-water vapour mixture is cooled at constant pressure, is called Dew Point (Figure 35.1)
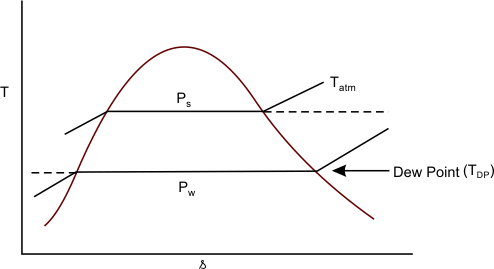
Figure 35.1
|