ADIABATIC SATURATION
Specific humidity or the relative humidity of an air – water vapour mixture can be measured in principle with the help of a device called the adiabatic saturator (see figure35.2).
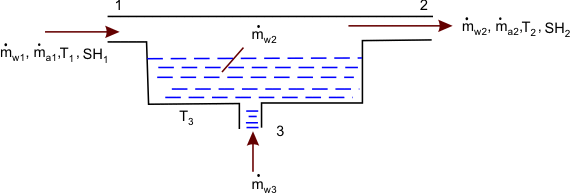
The air – water vapour mixture flows steadily into the device. The 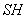 or or  of the incoming mixture has to be determined. of the incoming mixture has to be determined.
The air – water vapour mixture leaves the adiabatic saturator as saturated mixture. Let the device be insulated so that there is no energy loss.
Since the unsaturated air – water vapour mixture is sweeping over a layer of liquid water, some water evaporates. The energy needed for the evaporation comes from the air mixture. Hence, the air – water vapour mixture leaves the adiabatic saturator at a temperature lower than that of the entering air. As the air leaving the adiabatic saturator is in equilibrium with the liquid water, the temperature of the liquid water is equal to the temperature of the saturated air – water vapour mixture.
Mass balance for air
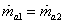 |
(35.16) |
Mass balance for water
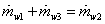 |
(35.17) |
Energy balance
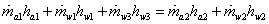 |
(35.18) |
Dividing (35.17) by 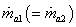 Dividing (35.18) by 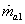
or
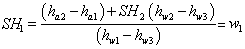 |
(35.20) |
The quantity
Another term, 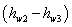 has to be estimated properly. has to be estimated properly.
Here 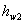 refer to the enthalpy of saturated water vapour at temperature refer to the enthalpy of saturated water vapour at temperature 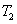 and and 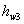 refers to the enthalpy of the saturated liquid at temperature refers to the enthalpy of the saturated liquid at temperature 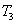 or or 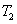 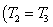 ) water pool temperature at steady state is same as ) water pool temperature at steady state is same as 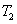 ) )
Therefore
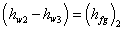 |
(35.21) |
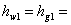 enthalpy of the saturated water vapour at state 1 enthalpy of the saturated water vapour at state 1
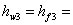 enthalpy of the saturated liquid water at temperature enthalpy of the saturated liquid water at temperature 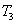 or or 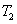 , , 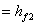
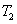 does not depend on the temp at which the liquid water enters the device (make-up water temperature) does not depend on the temp at which the liquid water enters the device (make-up water temperature)
The adiabatic saturation temperature 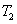 depends only on the conditions depends only on the conditions 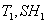 of the entering fluid. of the entering fluid.
Finally
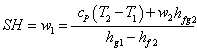 |
(35.22) |
|