Zeroth Law of Thermodynamics
Statement: If a body 1 is in thermal equilibrium with body 2 and body 3, then the body 2 and body 3 are also in thermal equilibrium with each other
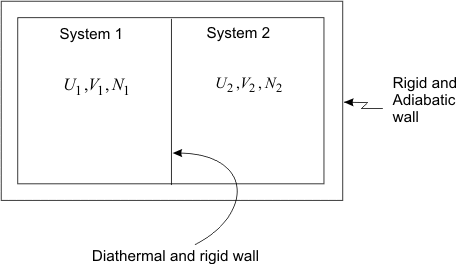
Figure 5.2
Two systems 1 and 2 with independent variables ( U1 , V1 , N1 ) and (U2 , V2 , N2 ) are brought into contact with each other through a diathermal wall (figure 5.2). Let the system 1 be hot and system 2 be cold. Because of interaction, the energies of both the systems, as well as their independent properties undergo a change. The hot body becomes cold and the cold body becomes hot. After sometime, the states of the two systems do not undergo any further change and they assume fixed values of all thermodynamic properties. These two systems are then said to be in a state of thermal equilibrium with each other. The two bodies which are in thermal equilibrium with each other have a common characteristic called temperature. Therefore temperature is a property which has the same value for all the bodies in thermal equilibrium.
Suppose we have three systems 1, 2 and 3 placed in an adiabatic enclosure as shown in figure 5.3.

Figure 5.3
The systems 1 and 2 do not interact with each other but they interact separately with systems 3 through a diathermal wall. Then system 1 is in thermal equilibrium with system 3 and system 2 is also in thermal equilibrium with system 3. By intuition we can say that though system 1 and 2 are not interacting, they are in thermal equilibrium with each other.
Suppose system 1 and 2 are brought into contact with each other by replacing the adiabatic wall by a diathermal wall as shown in figure 5.3 (B). Further they are isolated from system 3 by an adiabatic wall. Then one observes no change in the state of the systems 1 and 2.
|