Perfect Gas Scale
An ideal gas obeys the relation
P 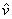 = R T = R T |
|
where R is the Universal Gas Constant ( R = 8.314 J/mol K). This equation is only an approximation to the actual behavior of the gases. The behavior of all gases approaches the ideal gas limit at sufficiently low pressure (in the limit P 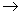 0). The perfect gas temperature scale is based on the observation that the temperature of a gas at constant volume increases monotonically with pressure. If the gas pressure is made to approach zero, the gas behavior follows the relation 0). The perfect gas temperature scale is based on the observation that the temperature of a gas at constant volume increases monotonically with pressure. If the gas pressure is made to approach zero, the gas behavior follows the relation
P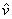 = R T = R T
Figure 5.4 shows a constant volume gas thermometer.
The bulb is placed in the system whose temperature is to be measured. The mercury column is so adjusted that the level of mercury stands at the reference mark S . This ensures that the volume of the gas is held at a constant value. Let the pressure of the gas be read as P. Let a similar measurement be made when the gas bulb is maintained at the triple point of water, Ptp. We can obtain triple point by putting water and ice in an insulated chamber and evacuating air ( which is then replaced by water vapour).
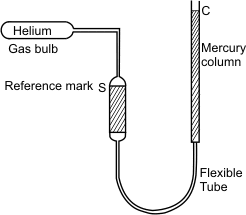
Figure 5.4
The temperature of the triple point of water has been assigned a value of 273.16 K. Since for an ideal gas T varies as P ,
or,
where Ttp is the triple point temperature of water.
Suppose a series of measurements with different amounts of gas in the bulb are made. The measured pressures at the triple point as well as at the system temperature change depending on the amount of gas in the bulb. A plot of the temperature Tcal , calculated from the expression T = 273.16 ( P/ P tp ) as a function of the pressure at the triple point, results in a curve as shown in figure 5.5.
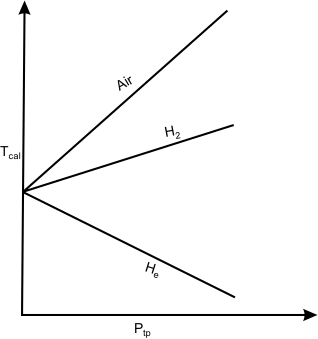
Figure 5.5
When these curves are extrapolated to zero pressure, all of them yield the same intercept. This behaviour can be expected since all gases behave like ideal gas when their pressure approaches zero. The correct temperature of the system can be obtained only when the gas behaves like an ideal gas, and hence the value is to be calculated in limit Ptp 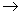 0. Therefore 0. Therefore
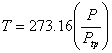 , as , as 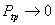 |
|
A constant pressure thermometer also can be used to measure the temperature. In that case,
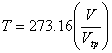 ; when ; when 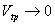 |
|
Here Vtp is the volume of the gas at the triple point of water and V is the volume of the gas at the system temperature.
|