First Law of Thermodynamics
The following are the observations during the experiment shown in Fig. 6.1.
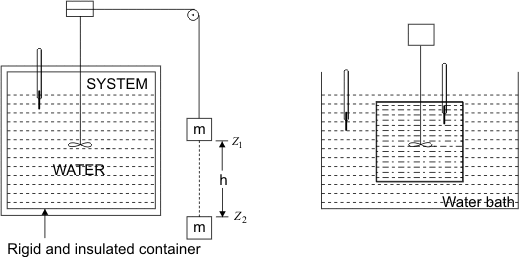
Figure 6.1
- Work done on the system by lowering the mass m through
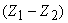 = change in PE of m = change in PE of m
- Temperature was found to increase
- System was brought into contact with a water bath
- System was allowed to come back to initial state
- Energy in transferred as heat from the system to the bath
The system thus executes as cycle which consists of work input to the system followed by the transfer of heat from the system.
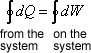 |
(6.1) |
Whenever a system undergoes a cyclic change, however complex the cycle may be, the algebraic sum of the work transfer is equal to the algebraic sum of the energy transfer as heat (FIRST LAW OF THERMODYNAMICS).
Sign convention followed in this text:
- Work done by a system on its surroundings is treated as a positive quantity.
- Energy transfer as heat to a system from its surroundings is treated as a positive quantity
 |
(6.2) |
or,
|