Specific Heat at Constant Pressure
Let us focus on Figure 7.3
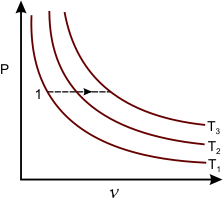
Figure 7.3
- System changes its state from 1 to 2 following a constant pressure process.
- There will be an accompanying change in temperature.
Specific heat at constant pressure is defined as the quantity of energy required to change the temperature of a unit mass of the substance by one degree during a constant pressure process.
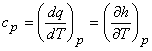 |
(7.9) |
The total heat interaction for a change in temperature from T1to T2 can be calculated from
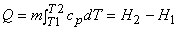 |
(7.10) |
The molar specific heat at constant pressure can be defined as
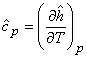 |
(7.11) |
|