Adiabatic Process
- Irreversible Adiabatic Process
A process in which there is no energy transfer as heat across the boundaries of the system, is called an adiabatic process. For an adiabatic process, Q=0. Paddle wheel work is performed on the system (Figure 7.5).
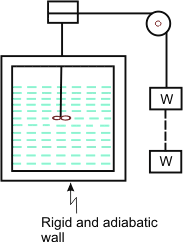
Figure 7.5
Application of first law gives
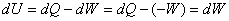 |
(7.15) |
or
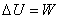 |
(7.16) |
In equ (7.16) W is the magnitude. However, the work is done on the system, hence it is a negative quantity.
- Reversible Adiabatic (ISENTROPIC) Process
Consider a gas contained in the cylinder piston assembly as the system. The cylinder wall and the piston act as adiabatic walls. Suppose the gas is allowed to expand from the initial pressure P1 to the final pressure P2 and the opposing pressure is so adjusted that it is equal to inside gas pressure. For such a process, dW=PdV
The first-law of thermodynamics will give
Let us consider the system as an ideal gas which satisfies the relation 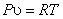 and and 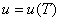 . Also, we know that . Also, we know that 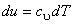 . .
or
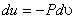 |
(7.17) |
or,
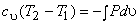 |
(7.18) |
or
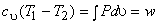 |
(7.19) |
or
For an ideal gas, 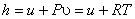
Therefore,
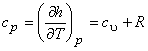 |
(7.20) |
The ratio of specific heats is given by
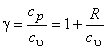 |
(7.21) |
or,
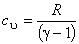 |
(7.22) |
Thus,
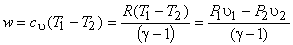 |
(7.23) |
Therefore when an ideal gas expands reversibly and adiabatically from the initial state 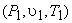 to final state to final state 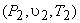 , the work done per mole of the gas is given by the above expression. , the work done per mole of the gas is given by the above expression.
|