Present Research Activities
The principal thrust of present research activities has been in the area of supramolecular chemistry of cryptands, synthesis and applications of coordination polymers/metal-organic frameworks and design and synthesis of chemosensors
(i) Supramolecular Chemistry of Cryptand
In the area of supramolecular chemistry of cryptand, significant contributions have been made to literature over the years.
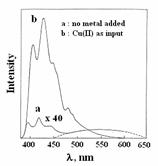
(a) A major contribution in this area is that transition metals as well as heavy metals, the known fluorescence quenchers, can induce large enhancement of fluorescence in cryptand-based fluorescence signaling systems.


Chem. Commun. (2005), 513.; J. Am. Chem. Soc. 119, (1997), 11903.; J. Am. Chem. Soc. 118, (1996), 1553.
(b) In a cryptand, metal ions always occupy the cavity inside. For the first time a metal ion was forced to translocate between inside and outside the cryptand cavity when electron-withdrawing groups are attached.
Inorg. Chem. 43, (2004), 4626.
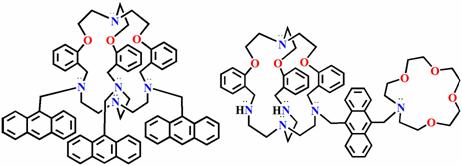
(c) Two new cryptands have been synthesized for the first time where Cu(II) ion move from one end to the other that can be monitored by fluorescence output. This is still the only example where a metal ion is shown to dance inside the cavity. This will be very useful in the area of molecular photonic devices for information processing.
Chem. Commun. (2008), 4180.
(d) Cryptand molecule has been derivatized with two/three different fluorophores. The enhancement is observed in the acceptor fluorophore in presence of transition metal ion, even when the donor fluorophore is excited due to substantial Förster Resonance Energy Transfer (FRET).
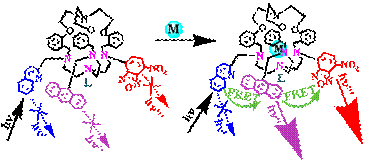
Chem. Commun. (2009), 4982.
(e) NLO activities have been probed on acyclic as well as macrobicyclic cryptands by femtosecond laser and theoretically at the B3LYP 6-31G* level.
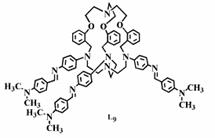
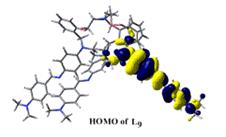
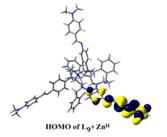
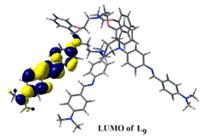
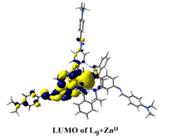
Chem. Eur. J. 14, (2008), 10628.
(f) Cryptands can be derivatized with long-chain alkyl groups to afford a new generation of amphiphiles. These amphiphiles form stable vesicles as well as Langmuir-Blodgett films.
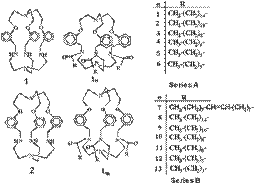
Langmuir 16, (2000), 1910.; Langmuir 14, (1998),7537.; Langmuir 14, (1998), 5712.; Langmuir 13, (1997), 3582.; Chem. Commun. (1996), 189.
(g) These laterally non-symmetric aza-cryptands of different dimensionality can be used for binding of various anions. Preferential encapsulation of one anion over another from a binary mixture of anions in the solid state has also been studied.
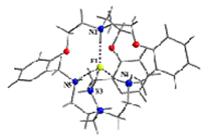
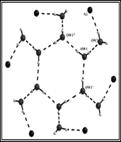
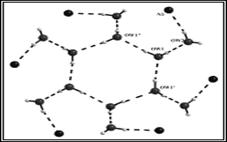
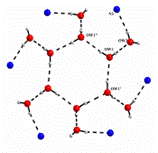
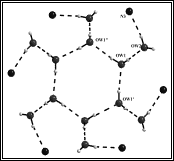
Cryst. Engg. Comm. 12, (2010), 2967.; Cryst. Engg. Comm. 12, (2010), 413.; Dalton. Trans. (2009), 6496.
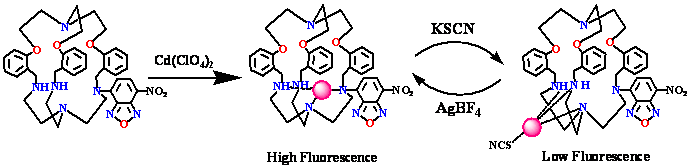
(h) Cryptand can be derivatized with rhodamine fluorophore to detect Hg(II) ion in aqueous medium selectively at ppb level. (Right) Fluorescence images of HEK 293 cells affected with Hg(II) seen through a confocal microscope.
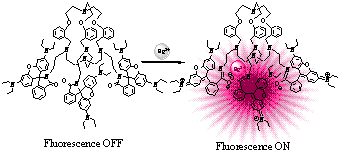
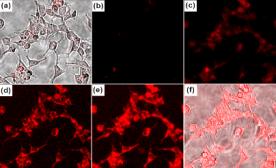
Chem. Commun. (2009), 4417.
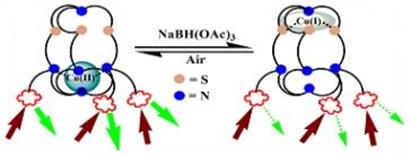
(i) Cryptand stabilized metal nanoparticles have been made. With Au nanoparticles both supraspgeres and eleongated dodecahedron can be stabilized that afford strong luminescence.
Chem. Commun. 54 (2018) 12836
(j) Cryptand stabilized Pd nanoparticles show excellent heterogeneous catalytic activity in the Suzuki-Miyaura reactions in water
Inorg. Chem. 58 (2019) 1003
Several other systems are being investigated which will be published later.
(ii) Metal Organic Frameworks
Research activities in this emerging area of chemistry involves synthesis of coordination polymers and use them to store gases of strategic importance, use some of these materials as heterogeneous catalysts, proton conductance and so on.
(a) A number of MOFs have been synthesized where the structures are strongly influenced by supramolecularly assembled water clusters and also proton conductance by metal organic frame work.
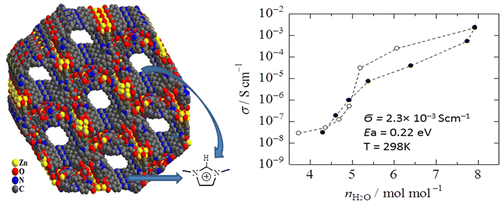
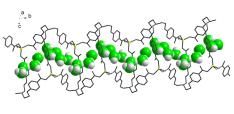
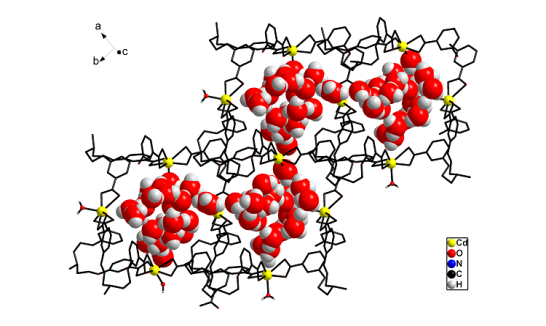
J. Am. Chem. Soc. (2012), 19432.; Angew. Chem. Int. Ed. 43, (2004), 3577.; Cryst. Growth Des. 6, (2006), 433.; Inorg. Chem. 44, (2005), 5553.; Inorg. Chem. 44, (2005), 3856.; Inorg. Chem. 44, (2005), 816.; Inorg. Chem. 43, (2004), 6887.; Inorg. Chem. 43, (2004), 5180.; Inorg. Chem. 43, (2004), 5495. 43.; Inorg. Chem. (2004), 2293.; Inorg. Chem. 43, (2004), 3771.; Inorg. Chem. 42, (2003), 8250.; Cryst. Eng. Commun. 6, (2004), 250.
(b) A few porous structures have been isolated that exhibit single-crystal to single-crystal (SC-SC) transformations and excellent catalytic activity in Knoevenagel condensation, cyanosilylation and Michael addition reactions. Geometrical isomers can also be separated as well as unstable intermediates can be stabilized using MOFs and also guest inclusion and structural transformation.
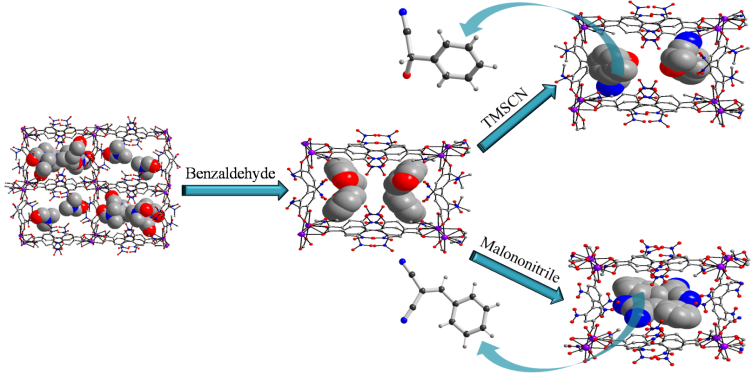
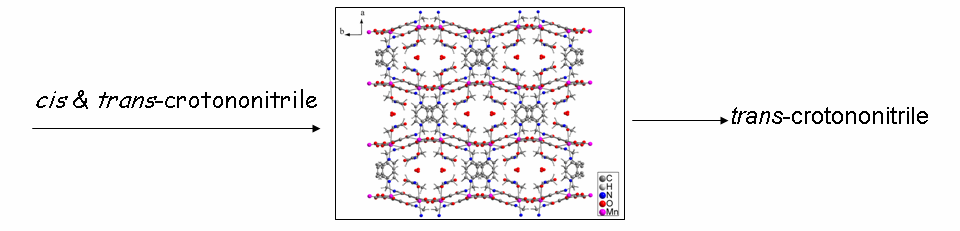
Chem. Eur. J. 18, (2012), 6866.; Chem. Eur. J. 16, (2010), 5070.; J. Am. Chem. Soc. 131, (2009), 10942.; Eur. J. Inorg. Chem. (2010), 3829.
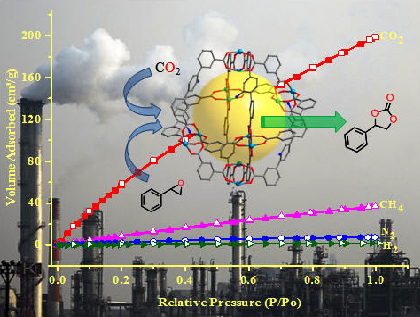
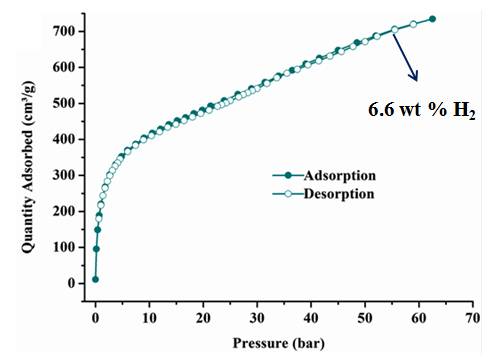
(c) Adsorption of selected gases and vapors by MOFs is an area of intense research activity at present. Several such systems have been synthesized and studied. These are showing excellent methane, carbon dioxide, hydrogen adsorption properties.
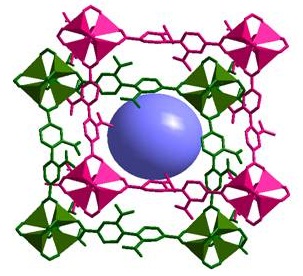
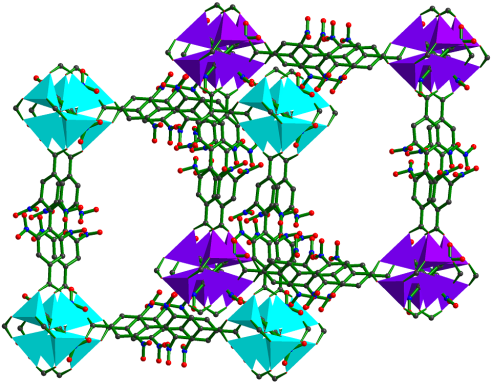
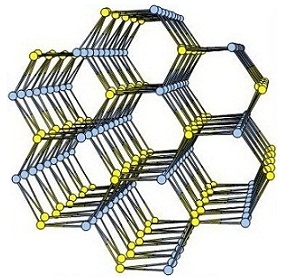
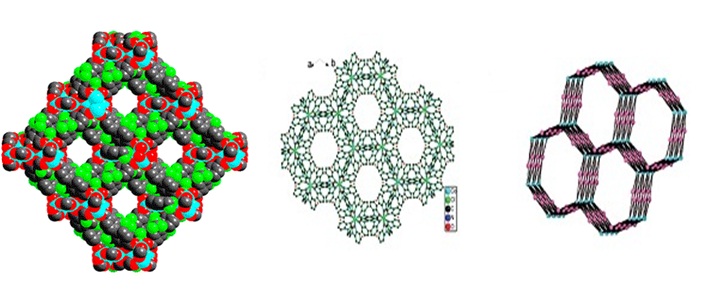
Inorg. Chem. 53 (2014), 7591, Dalton Trans. 2014, 6100, Inorg. Chem. 50, (2011), 539. Inorg. Chem. 52 (2013), 7358.; Cryst. Growth Des. 12, (2012), 2999.; Cryst. Growth Des. 13, (2013), 1238.; Cryst. Growth Des. 10, (2010), 3410.
(d) Crystal dynamics is an important phenomenon in MOFs. We have shown bicycle pedal motion in crystals.
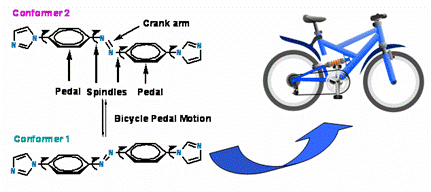
Inorg. Chem. 50, (2011), 1889; Cryst. Growth Des. 12 (2012) 5025.
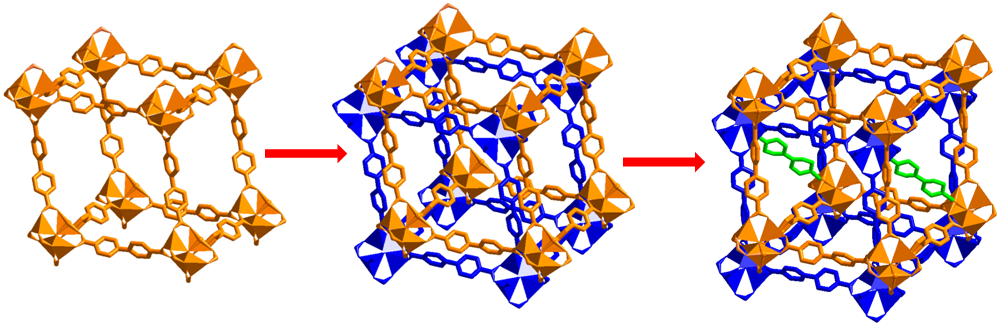
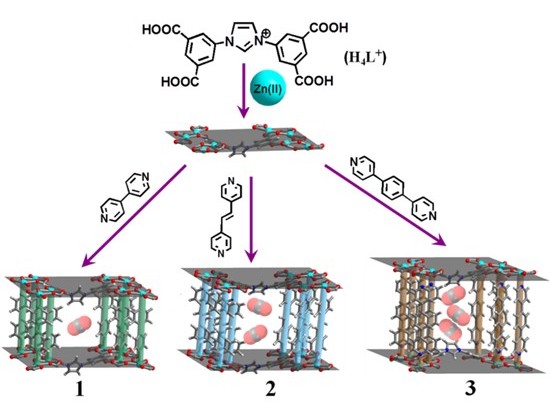
(e) Single-crystal to single-crystal transformations in MOF is a relatively recent phenomenon. It allows for direct observarion of changes via X-ray crystallography. It also allows exchange of metals as well as ligands to arrive at a structure not possible to synthesize directly. Some of these are illustrated below.
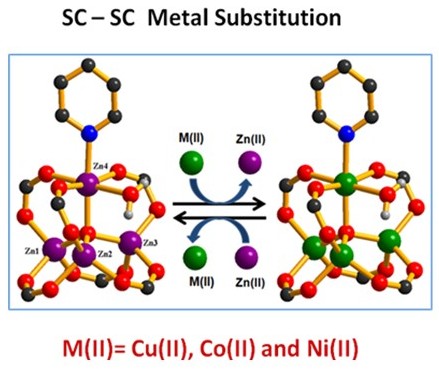
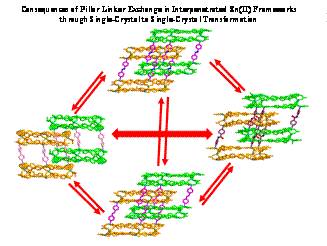
Encyclopedia of Inorganic and Bioinorganic Chemistry; Book Chapter. Wiley-Blackwell, John Wiley & Sons Inc., 1st Edition, 2014 (ISBN: 9781119951438) (in press), Cryst. Growth Des. (2014) 3623; Cryst Eng. Comm. (Highlights article). (2013) 9239, Chem. Eur. J. (2015), 16083, Chem. Eur. J. (2015), 17422, Chem. Eur. J. (2015), 21, 19064, Chem. Commun. (2015), 51, 3173, Chem. Commun. (2017), 53, 13371.
Chem. Eur. J. (2015), 21 17422. Chem. Commun. (2017), 53, 13371
Several fundamental problems like nature of the cavity, their shapes and sizes, area of p surfaces, interpenetrated structures, effects of loading and temperature on selectivity of gas absorption, etc. are being probed. We intend to explore MOFs for gas adsorption and heterogeneous catalysis. Our research efforts are expected to be of relevance in the production of many chemicals as well as supramolecular storage of energy-rich gas molecules.
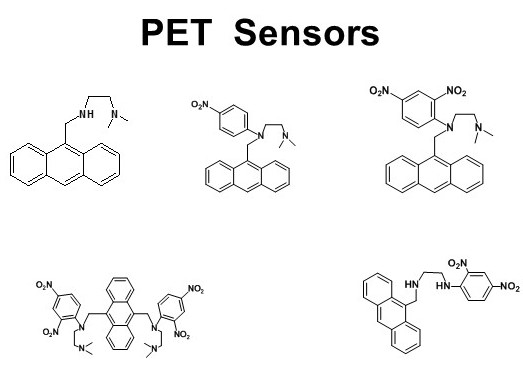
(III) Chemosensors with acyclic systems
A large number of acyclic chemosensors for different metal ions have been designed based on different mechanisms such as PET, ESIPT, spirolactum ring opening, restricted rotation, and so on. A few are shown here.
Inorg. Chem. 2015, 54, 8, 3929-3936
J. Phys. Chem. B 109, (2005), 4377.
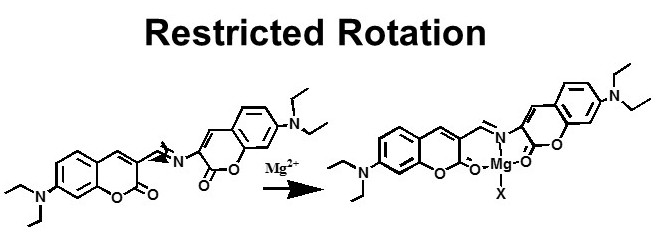
Inorg. Chem., (2008), 47, 2252
An ESIPT sensor for Hg2+ ion
Dalton Trans., 2015, 44, 20139-20146
Spirolactum ring opening system for Al3+ ion
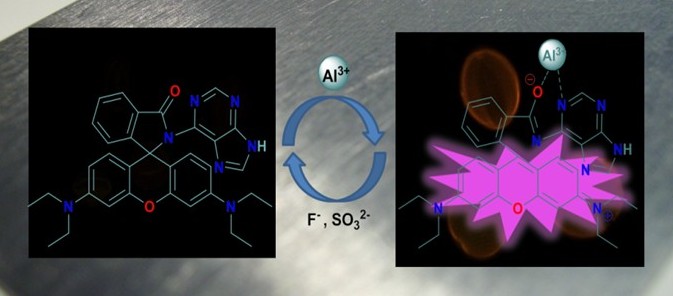
J. Luminescence, (2016) 169, 334
An acyclic ligand designed for High TPA in presence of Zn2+ ion
J. Am. Chem. Soc., (2006), 128, 402