Principle of increase of entropy
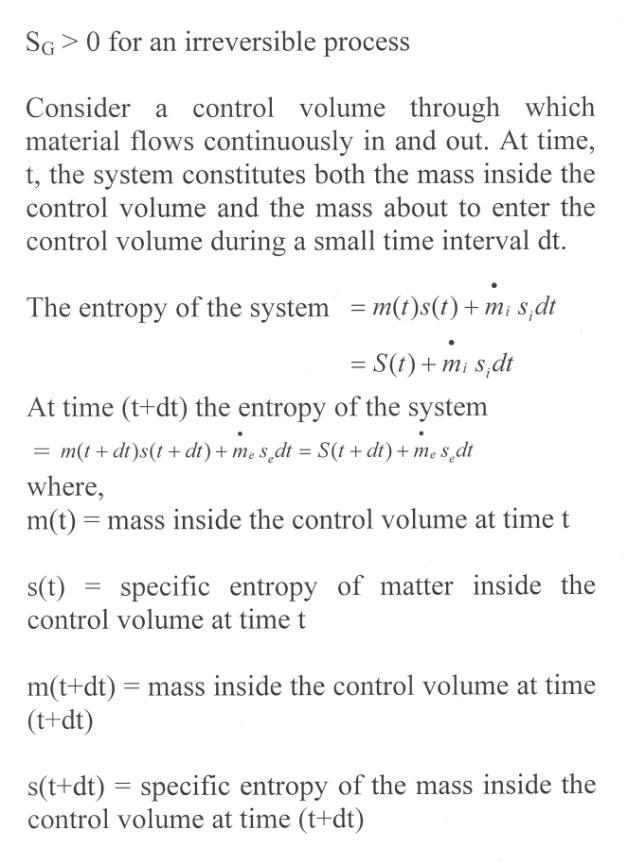
Let a system change from state 1 to state 2 by a reversible process A and return to state 1 by another reversible process B. Then 1A2B1 is a reversible cycle. Therefore, the Clausius inequality gives:
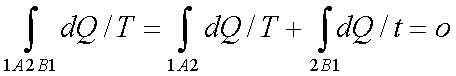
If the system is restored to the initial state from 1 to state 2 by an irreversible process C, then 1A2C1 is an irreversible cycle. Then the Clausius inequality gives:
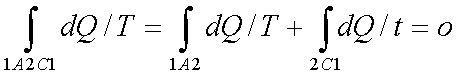
Subtracting the above equation from the first one,
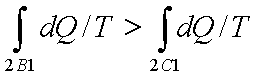
![]()
Since the process 2B1 is reversible,
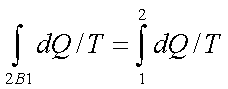
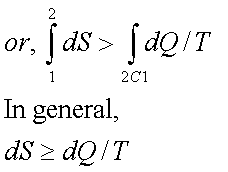
Where the equality sign holds good for a reversible process and the inequality sign holds good for an irreversible process.
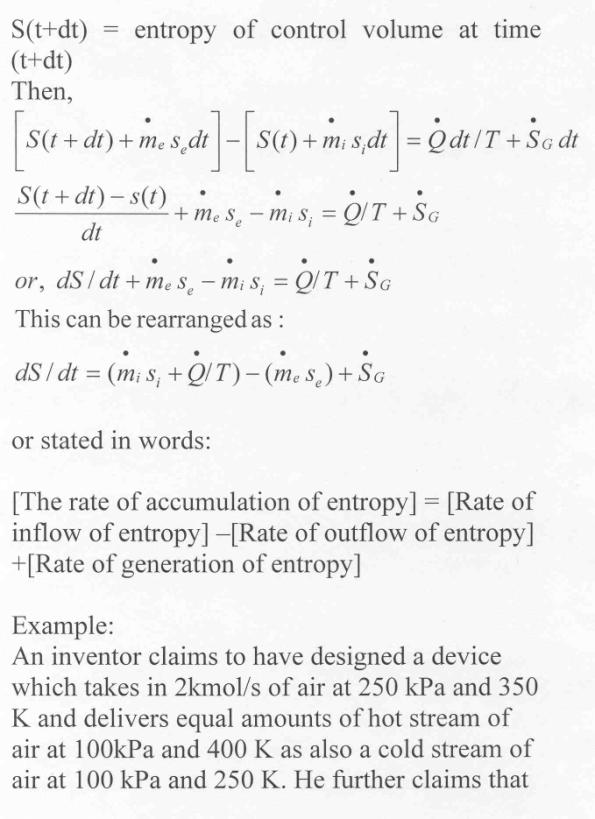
Now let us apply the above result to evaluate the entropy change of the universe when a system interacts with its surroundings and exchanges energy as heat with the surroundings.
Let Tsur and Tsys
be the temperatures of the surroundings and the system such that Tsur >Tsys. Let dQ represent the energy transfer as heat
from t he surroundings to the system during the given irreversible process.
dSsys = dQ/Tsys
dSsur = -dQ/Tsur
dSuni = dSsys + dSsur =
(dQ/T)sys – (dQ/T)sur >0
DSuni >0 (since
Tsur>Tsys)
If the system is isolated,
there is no change in the entropy of the surroundings and
DS ³ 0, for an isolated system
Therefore the entropy of an
isolated system either increases or, in the limit, remains constant.
The equality sign holds good
when the process undergone by the system is reversible, the inequality sign
holds good if there is any irreversibility present in the process. This
statement is usually called the principle of entropy increase.
Irreversible or spontaneous processes can occur only in that direction for which the entropy of the universe or that of an isolated system, increases. These processes cannot occur in the direction of decreasing entropy.
For an isolated system,
DS > 0, for
irreversible processes
DS = 0, for
reversible processes
DS < 0, the
process is impossible
Example:
One kg of superheated steam
at 0.2MPa and 2000C contained in a piston cylinder assembly is kept
at ambient conditions of 300K till the steam is condensed to saturated liquid
at constant pressure. Calculate the change in the entropy of the universe with
this process.
Solution:
Initial state of the steam:
superheated at 0.2 MPa and 200oC
h1= 2870.4 kJ/kg;
and s1 = 7.5033 kJ/kgK
Final state: saturated
liquid at 0.2 MPa.
h2 = 504.52 kJ/kg
and s2 = 1.5295 kJ/kgK
Hence DSsteam = s2 – s1 = 1.5295 – 7.5033 =
-5.9738 kJ/kgK
For a constant pressure process:
q = Dh
Therefore, q = h2
– h1 = 504.52 – 2870.4 =
-2365.68 kJ
Entropy change of the surroundings = DSsur = Q/Tsur
= 2365.88/300 = 7.886 kJ/K
Hence, DSuni = DSsys + DSsur = -5.9738
+7.886 = 1.9122 kJ/K
DSuni > 0 and
hence the process is irreversible and feasible.
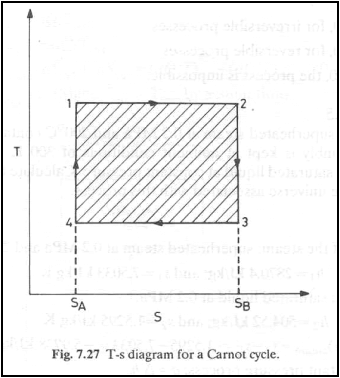
Temperature-Entropy diagram
Entropy change of a system
is given by dS = (dQ/T)R. during energy transfer as heat to the
system from the surroundings is given by
dQ = TdS. Hence if T and S
are chosen as independent variables, then the integral ![]() is the area under the curve.
is the area under the curve.
The first law of
thermodynamics gives
dU = dQ - dW
also for a reversible process,