The Van der Waals Constants
The constants 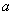 and and 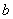 are different for different substances. We can get the estimates of are different for different substances. We can get the estimates of 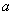 and and 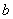 of a substance by knowing the critical values of that substance. of a substance by knowing the critical values of that substance.
Before we proceed further, let us look at the mathematical function 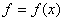
For the maximum value of x: 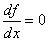 and and 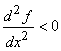
For the minimum value of x: 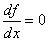 and and 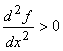
For the point of inflection, 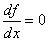 and and 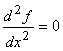
From the Figure 9.2 we can see that the critical point is a point of inflection.
The critical isotherm must show a point of inflection at the critical point
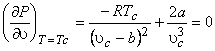
|
(10.1) |
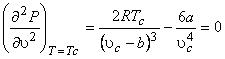 |
(10.2) |
At the critical point, the Van der Waals equation is
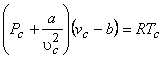 |
(10.3) |
One can solve the above three equations to obtain
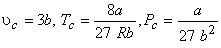 |
(10.4) |
Alternatively, one can calculate the Van der Waals constants 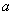 and and 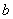 in terms of critical constants. in terms of critical constants.
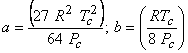 |
(10.5) |
The constants 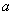 and and 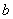 can be determined for any substance from the critical point data (Table 10-1) At the critical point, one can see that can be determined for any substance from the critical point data (Table 10-1) At the critical point, one can see that
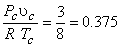 |
(10.6) |
Table 10.1 Some typical values of Van der Waals constants |
Gas |
Constants 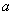
kPa (m3/ kmol)2
|
Constants 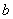
( m3/ kmol ) |
Air
|
135.8 |
0.0364 |
Ammonia (NH3 )
|
423.3 |
0.0373 |
Carbon-dioxide(CO2 )
|
364.3 |
0.0427 |
Hydrogen (H2 )
|
24.7 |
0.0265 |
Oxygen (O2 )
|
136.9 |
0.0315 |
Nirtogen (N2 ) |
136.1 |
0.0385 |
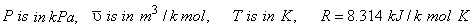
|