Virial Equations of State
For any gas, we can write
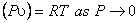 |
(10.7) |
or,
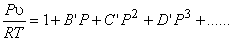 |
(10.8) |
An alternate expression is
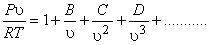 |
(10.9) |
Both expressions are known as virial expansions introduced by Kamerlingh Onnes. 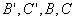 etc are called virial coefficients. etc are called virial coefficients. 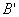 and and 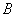 are called second virial coefficients, are called second virial coefficients, 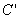 and and 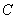 are called third virial coefficients and so on. For a given gas, these coefficients are functions of temperature only. are called third virial coefficients and so on. For a given gas, these coefficients are functions of temperature only.
|