Pure Substance
A pure substance is one which consists of a single chemical species.
Concept of Phase
Substances can be found in different states of aggregation. Ice, water and steam are the three different physical states of the same species  . Based on the physical states of aggregation the substances are generally classified into three states as . Based on the physical states of aggregation the substances are generally classified into three states as
- Solid
- liquid
- gas
The different states of aggregation in which a pure substance can exist, are called its phases. Mixtures also exist in different phases. For example, a mixture of alcohal and water can exist in both liquid and vapour phases. The chemical composition of the vapour phase is generally different from that of the liquid phase.
Generalizing the above observations, it can be said that a system which is uniform throughout both in chemical composition and physical state, is called a homogeneous substance or a phase.
All substances,-solids, liquids and gases, change their specific volume depending on the range of applied pressure and temperature.
When the changes in the specific volume is negligible as in the case of liquids and solids, the substance may be treated as incompressible.
The isothermal compressibility of a substance κ is defined as
The coefficient of volume expansion of a substance 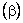 is defined as is defined as
|