Equations of State
Equations that relate the pressure, temperature and specific volume or molar volume of a substance. They predict the 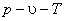 relationship of a “gas” reasonably well within selected regions. relationship of a “gas” reasonably well within selected regions.
Boyle's law:
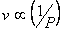 at constant pressure at constant pressure |
|
or,
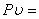 Constant at constant temperature Constant at constant temperature |
|
Charles' Law:
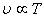 At constant pressure At constant pressure |
|
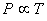 At constant volume At constant volume |
|
Boyle's law and Charles' law can be combined to yield
Where R is the characteristic gas constant. If the specific volume 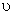 is substituted by the molar volume, is substituted by the molar volume, 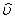 then R is substituted by the universal gas constant, then R is substituted by the universal gas constant, 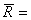 8.314 kJ / kmol K. Again, it can be shown that for an ideal gas 8.314 kJ / kmol K. Again, it can be shown that for an ideal gas 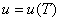 . .
In summary, the Ideal gas equation:
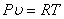 where R is the specific constant where R is the specific constant |
|
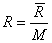 , where , where 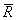 is the universal gas constant (8.314 kJ / k mole K) M is the molar mass, defined as the mass of one mole of a substance. We can also write is the universal gas constant (8.314 kJ / k mole K) M is the molar mass, defined as the mass of one mole of a substance. We can also write 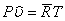 where where 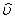 is the molar volume. is the molar volume.
Example: M = 28 kg / kmol for nitrogen (since its molar mass is 28)
M =32 kg / kmol for oxygen ( O2 with a molar mass of 32)
Note: Many gases such as air, oxygen can be treated as ideal gases. However, dense gases such as water vapor and refrigerant vapor should not be treated as ideal gas. Use property table instead.
|