Bera group studies synthetic and mechanistic organometallic chemistry. Our research involves designing ligands featuring desired features/functionalities, synthesis and reactivity of new organometallic compounds, and the rational development of organometallic catalysts for the activation, functionalization and conversion of challenging substrates to useful chemicals. We are also interested in the use of these compounds in biology and material chemistry. Currently, research is focused on the following areas: Synthesis and Reactivity of Organometallic Compounds Small Molecule Activation Direct Air Capture and Conversion of CO2
Ligand Design
Innovative ligand design is at the heart of developing tailored metal complexes with specific structure and reactivity. Constructing new ligands featuring desired functionalities is the main activity of our group. In particular, we have been fascinated by the unique stereo-electronic properties of the naphthyridine-functionalized annulated and non-annulated NHCs and MICs. Many of our fabricated ligands are capable of actively participating in catalysis by undergoing reversible changes during the catalytic cycle.
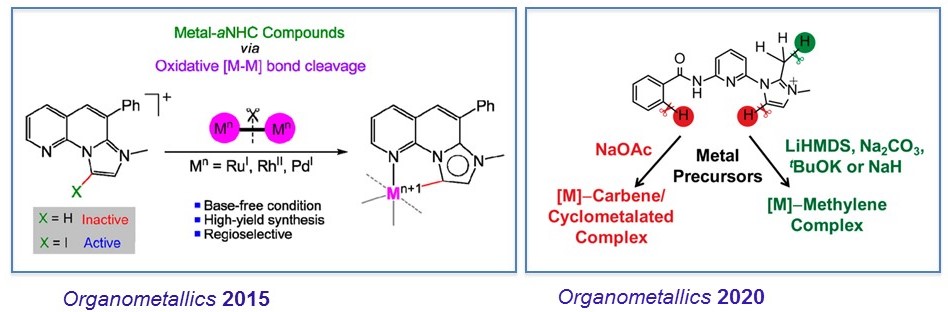
We work on the design, synthesis and reactivity of new organometallic complexes. Our group reported a base-free method for accessing metal−mesoionic carbene (MIC) compounds from metal−metal bonded bimetallic precursors and imidazolium salts. Recently, we developed a base-controlled directed synthesis of metal–methyleneimidazoline (MIz) and Metal–MIC compounds.
We have designed catalysts featuring pendant basic functional groups (pyridyl- or 1,8-naphthyridyl), which engage in secondary interactions with water, thus assisting its nucleophilic attack to a metal-bound substrate. Using this design principle, we demonstrated oxygenation of the Ir-bound cyclooctadiene and developed effective Ru(II), Rh(I) and Au(I) catalysts for the conversion of alcohols to carboxylic acid, organonitriles to amides and the alkynes to amides, respectively. We also explored the hemilabile basic group for water activation to overcome the limitations posed by a ligand design based on a free basic group. The ongoing studies focus on fabricating 3d metal-based catalysts bearing proton-responsive functionalities on the ligand scaffold for a wide variety of challenging hydrogenation and dehydrogenation reactions. Protic moieties act as a source or sink of a proton in the proton/hydride transfer reactions, thereby enabling easily accessible, alternate mechanistic pathways to that of classical catalysts devoid of such functionalities. Our catalyst design is driven by exhaustive mechanistic studies of the catalytic transformations using experimental and computational methods.
The current state-of-the-art in the capture of CO2 from the atmosphere, its storage and subsequent hydrogenation to MeOH is unable to mitigate the increase in the CO2 amount caused by the burning of fossil fuels. We aim to develop methodologies for the integrated capture and hydrogenation of CO2 to methanol using protic catalysts. In this novel approach, the proton-responsive unit in the protic catalyst not only assists the hydrogenation of CO2 capture products but facilitates the capture of CO2 as well.