New Antimicrobials
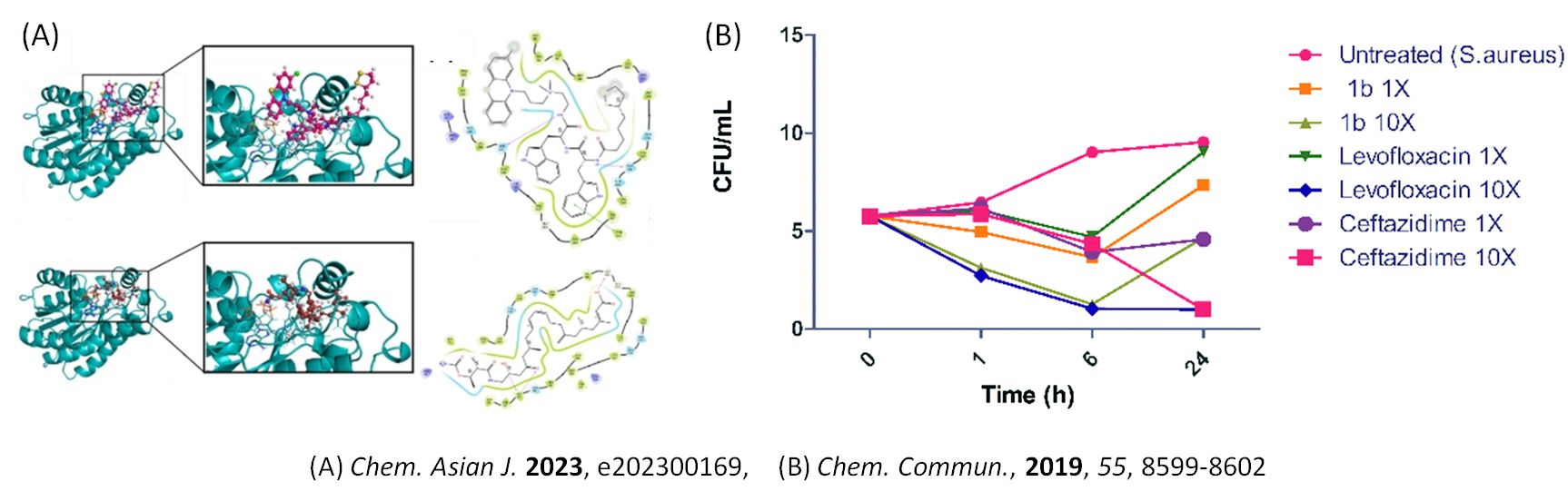
We are interested in developing an effective strategy to fight against multi-drug resistant bacteria using peptide-based antibiotics, like AMPs, a divergent class of membrane-permeating peptides with crucial functions in innate host defense. AMPs (Antimicrobial peptides) are organic molecules produced by various multicellular living organisms and are responsible for innate immunity. They have the potential to be used as antibiotics considering a number of advantages they have to offer as compared to traditional antibiotics. We aim to establish the fundamental biotechnology principles for developing a new class of peptide antimicrobials by using innovative strategies, aiming to establish principles of a priori design of AMPs by a combination of empirical methods and machine learning-driven drug discovery to explore means of improving pharmacokinetic properties of AMPs by the use of co-crystallization, derivatization with unnatural amino acids and enclosure by nanostructured delivery matrices.
Ordered Peptide Assemblies
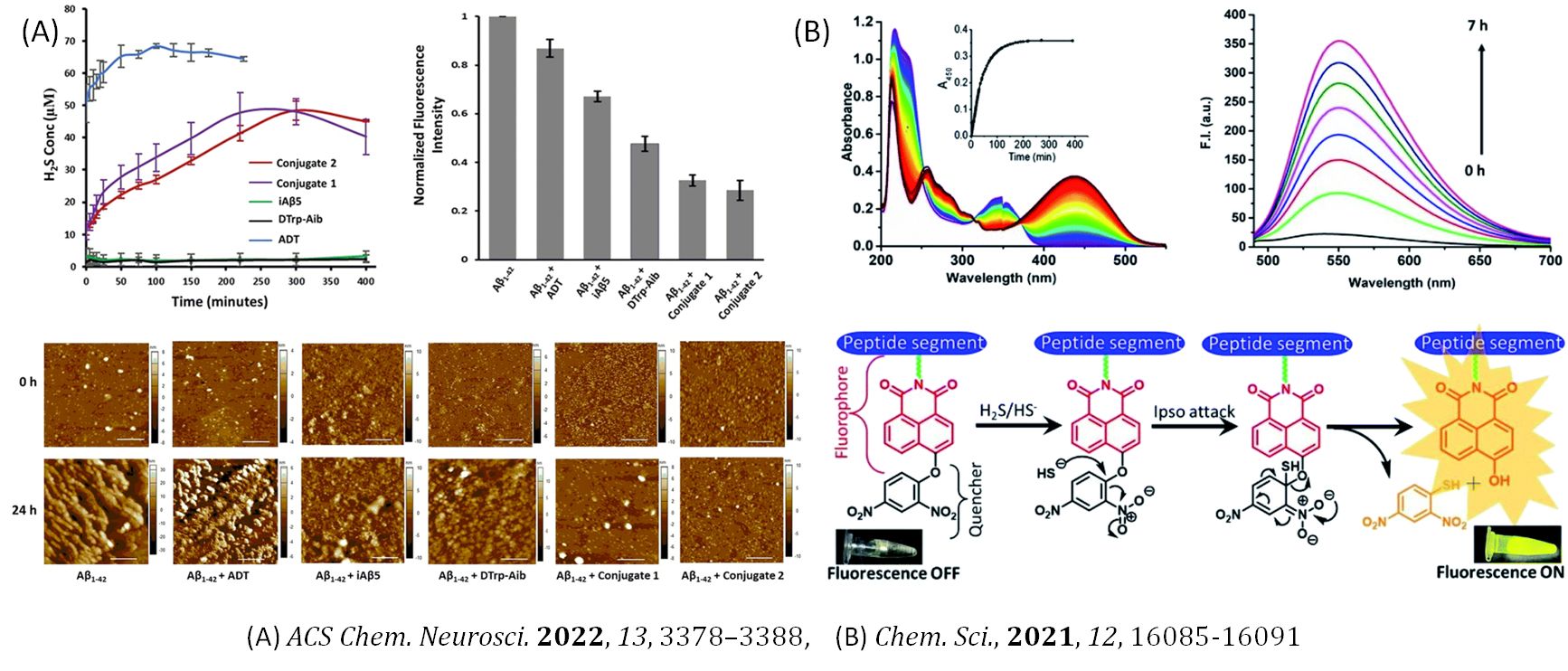
Ordered peptide assemblies form a range of assemblies from simple linear arrangements to complex three-dimensional structures, which allows precise control over their properties and functions. By manipulating their self-assembly behavior, one can achieve highly organized functional materials with tailored properties, such as mechanical strength, specificity, spontaneity, stability, and biocompatibility. Due to its diverse applications in biotechnology and medicine, we are interested in the peptide assemblies with novel antibacterial compounds, fluorescent probes, gasotransmitter delivery molecules, stimuli-sensitive morphologies, anti-cancer molecules, cell sorting platforms, anti-amyloidogenic compounds, and many other therapeutic molecules to ameliorate diverse pathophysiological conditions.
Small Molecule Stem Cell Interaction
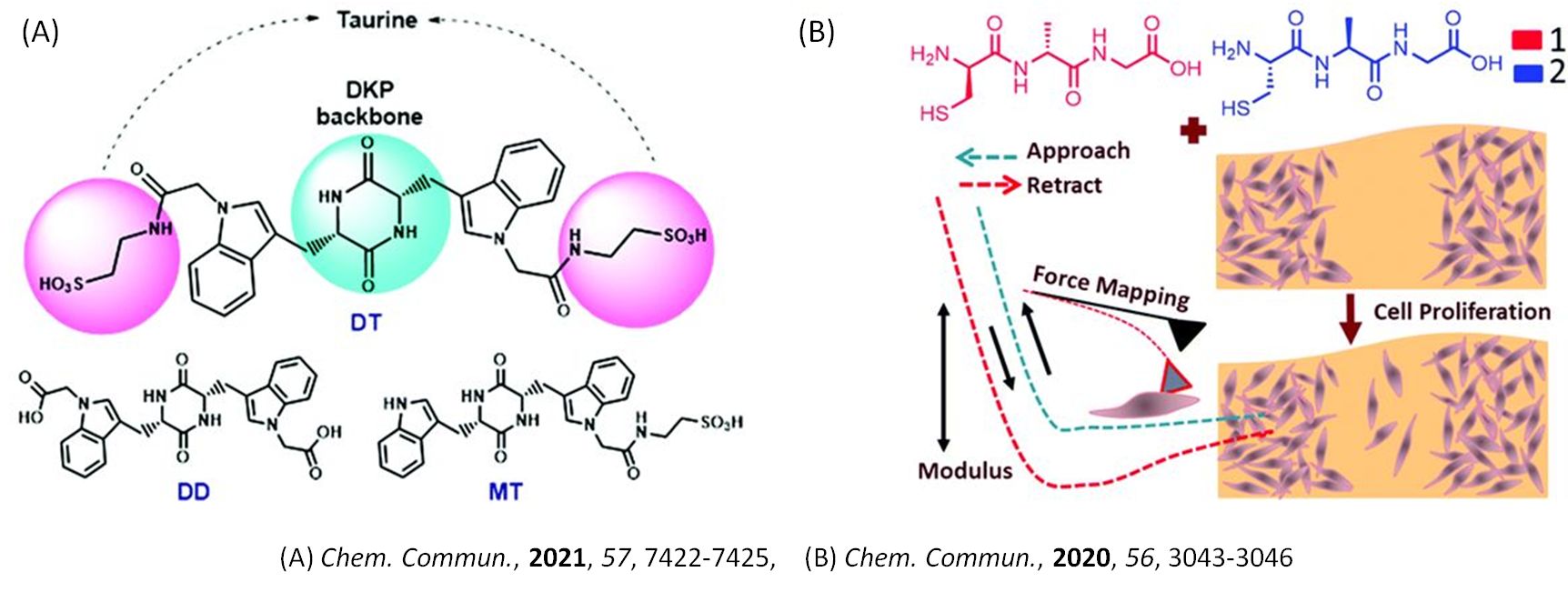
Currently, we are exploring small bioactive molecules like peptides and nucleobases and their ability to proliferate and differentiate mesenchymal stem cells (MSCs) into cell lineages, specifically bone cells. MSCs being multipotent and self-renewal, play a pivotal role in tissue engineering and regenerative medicine. Small bioactive molecules that trigger osteogenic differentiation of MSCs via activating many biological signalling pathways are of great importance and offer great promise for the new therapeutics of bone-related disorders. Recently we reported two peptides that strongly enhanced proliferation and cellular elasticity and finally promoted wound healing in human MSCs (hMSCs) without stimulating osteogenic and adipogenic differentiation. In another work, we reported a tryptophan-based novel diketopiperazine that induced osteogenic differentiation of murine pre-osteoblast cells and bone-marrow-derived stem cells in vitro and 3D composite cryogel. Such functionalised diketopiperazine can serve as potential scaffolds for bone healing and regeneration.
Self-Assembled Nucleobases
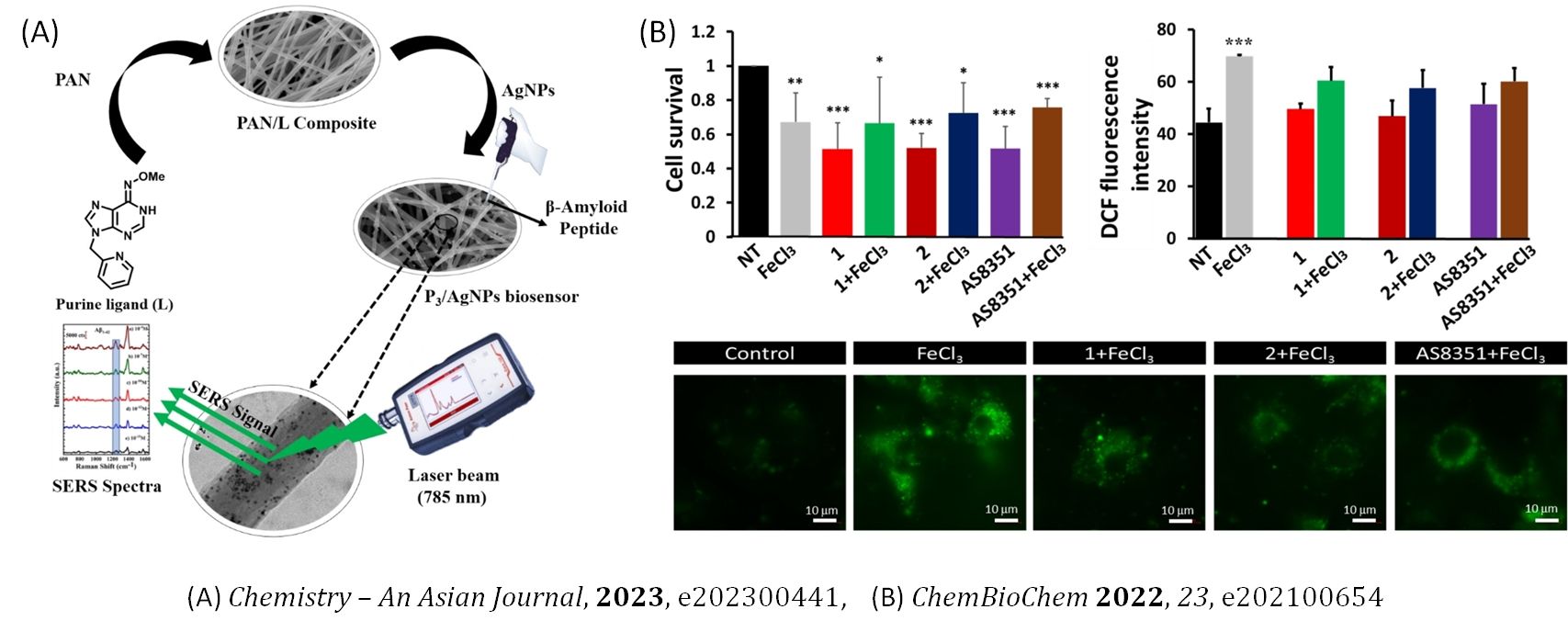
Nucleobases, due to their ability to engage in hydrogen bonding, π-π stacking for stabilisation of higher-order structures, and their self-assembled structures support structural diversity for therapeutic applications. We are interested in understanding the self-assembly of nucleobase molecules and their synthesis for specific screening of neurodegenerative biomarkers and the development of biosensors using electrospinning and microfluidics. We are also interested in exploring the metal-ion coordination, stabilising higher-order superstructures of these molecules along with the generation of interesting supramolecular structural paradigms when bestowed with suitably predisposed metal ion binding sites and their implication for a variety of biological functions.