(a)
Pressure-Temperature Diagram
For water , at 100 kPa, the saturation temperature is 99.60C. Alternatively at 99.60C, the saturation pressure is 100 kPa. Quite often, the saturation pressure is called the vapour pressure.
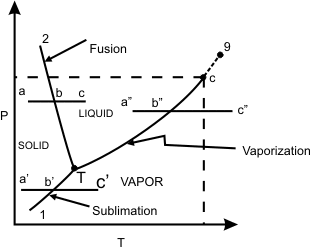
Figure 11.1
Refer to figure 11.1, The segment 1-T represents sublimation process during which solid and vapour phases coexist in equilibrium. The segment 2-T represents fusion process during which solid and liquid phases coexist in equilibrium. The segment T-C represents vapourization .
Sublimation curve 1-T separates solid and vapour. 2-T separates solid and liquid and T-C separates liquid and vapour. Three curves meet at T, which is called the triple-point, where all the three phases-solid, liquid and vapour coexist in equilibrium. At the triple point no thermodynamic property of the system can be varied independenty. The system is said to be invariant.
Along, 1-T, or T-C, the system is univariant , that is only one thermodynamic property of the system can be varied independently. The system is bivariant in the single phases region.
The curve 2-T can be extended indefinitely, the curve T-C terminates at point C which is called the critical point .
The critical point represents highest temperature and pressure at which both the liquid phase and vapour phase can coexist in equilibrium.
At the critical point , the specific volumes and all other thermodynamic properties of the liquid phase and the vapour phase are indentical.
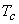 and and 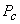 are called the critical temperature and critical pressure, respectively. If the substance exist as a liquid on the curve T−C, it is called saturated liquid, and if it exists as a vapour, it is called a saturated vapour. Under the constant pressure, the line abc indicates melting, are called the critical temperature and critical pressure, respectively. If the substance exist as a liquid on the curve T−C, it is called saturated liquid, and if it exists as a vapour, it is called a saturated vapour. Under the constant pressure, the line abc indicates melting, 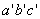 - sublimation and - sublimation and 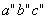 - vaporization. - vaporization.
|