(b)  Diagram Diagram
Refer to the  diagram as shown in Figure 11.2. diagram as shown in Figure 11.2.
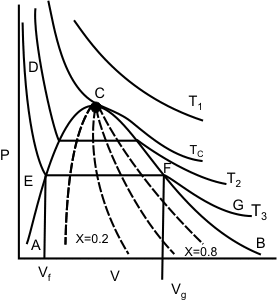
Figure 11.2
The isotherm 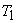 is at a temperature greater than the critical temperature is at a temperature greater than the critical temperature 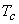 . The isotherms . The isotherms 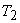 and and 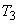 are at temperatures less than the critical temperature and they cross the phase boundary. The point C represents the critical point. The curve AC is called the saturated liquid line; and the curve CB is called the saturated vapour line. The area under the curve ACB is the two-phase region where both liquid and vapour phase are present. Left to the curve AC is the liquid region. Region to the right of curve CB is the vapour region are at temperatures less than the critical temperature and they cross the phase boundary. The point C represents the critical point. The curve AC is called the saturated liquid line; and the curve CB is called the saturated vapour line. The area under the curve ACB is the two-phase region where both liquid and vapour phase are present. Left to the curve AC is the liquid region. Region to the right of curve CB is the vapour region
The isotherm 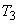 appears in three segments: DE, EF and FG. DE is almost vertical, because the change in the volume of liquid is very small for a large change in pressure. The segment FG is less steep because vapour is compressible. Segment EF is horizontal, because the phase change from liquid to vapour occurs at constant pressure and constant temperature for a pure substance. EF represents all possible mixtures of saturated liquid and saturated vapour. The total volume of the mixture is the sum of the volumes of the liquid and vapour phases. appears in three segments: DE, EF and FG. DE is almost vertical, because the change in the volume of liquid is very small for a large change in pressure. The segment FG is less steep because vapour is compressible. Segment EF is horizontal, because the phase change from liquid to vapour occurs at constant pressure and constant temperature for a pure substance. EF represents all possible mixtures of saturated liquid and saturated vapour. The total volume of the mixture is the sum of the volumes of the liquid and vapour phases.
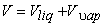 |
(11.1) |
Dividing by the total mass m of the mixture, m an average specific volume for mixture is obtained
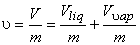 |
(11.2) |
Since the liquid phase is a saturated liquid and the vapour phase is a saturated vapour,
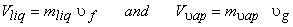 |
(11.3) |
so,
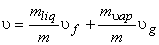 |
(11.4) |
Introducing the definition of quality or dryness fraction 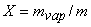 and noting that and noting that 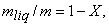 the above expression becomes the above expression becomes
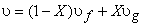 |
(11.5) |
The increase in specific volume due to vaporization 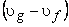 is often denoted by is often denoted by 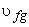
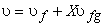 |
(11.6) |
Again we can write
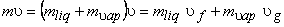 |
(11.7) |
or,
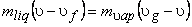 |
(11.8) |
or,
 |
(11.9) |
Known as lever rule. At the critical point, the specific volumes and all other thermodynamic properties of the liquid phase and the vapour phase are identical.
|