Now let us apply the above results to evaluate the entropy of the universe when a system interacts with the surroundings. Refer to Figure 23.2.
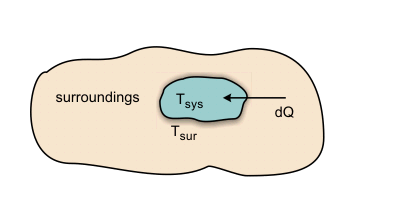
Figure 23.2
Let Temperature of the surroundings 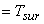 Temperature of the system 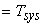
and,
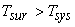 |
(23.7) |
If the energy exchange takes place, 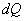 will be the energy transfer from the surroundings to the system. will be the energy transfer from the surroundings to the system.
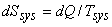 |
(23.8) |
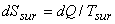 |
(23.9) |
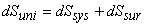 |
(23.10) |
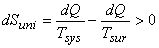 [since [since 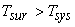 ] ] |
(23.11) |
So,
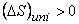 |
(23.12) |
If the system is isolated, that is, when there is no interaction between the system and the surroundings, then
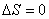 |
(23.13) |
|