Now let us redefine a system which includes our earlier system and its surroundings. Then
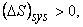 for an isolated system for an isolated system |
(23.14) |
Through generalization we can write for an isolated system
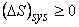 |
(23.15) |
Examine the statement critically. For a reversible process it is equal to zero. For an irreversible process it is greater than zero.
Similarly it is possible to write 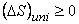 . The equality sign holds good if the process undergone is reversible; the inequality sign holds good if the process undergone is irreversible. . The equality sign holds good if the process undergone is reversible; the inequality sign holds good if the process undergone is irreversible.
Clausius summarized these as:
- Die Energie der Welt ist konstant.
- Die Entropie der Welt strebt einem Maximum
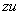 . .
This means:
- The energy of the universe in constant (first law).
- The entropy of the universe tends towards a maximum (second law)
|