| |
Steady-state steady-flow processes
The above equation can be simplified if one assumes steady flow and steady state conditions. Under these conditions, there is no change in the entropy of the control volume with respect to time, that is 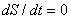 . The steady flow assumption implies that . The steady flow assumption implies that 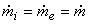 and that and that 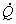 is a constant. Then we can write, is a constant. Then we can write,
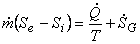 |
(24.20) |
or,
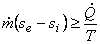 |
(24.21) |
For an adiabatic process 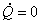 therefore therefore 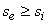 . However, if the process is reversible and adiabatic then . However, if the process is reversible and adiabatic then 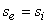
Summary
In this lecture we have shown that
- The Carnot cycle on a T − S diagram and identify the heat transfer at both the high and low temperatures, and the work output from the cycle.
- 1-2, reversible isothermal heat transfer
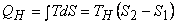 area 1-2-B-A. area 1-2-B-A.
- 2-3, reversible adiabatic expansion isentropic process, S = constant
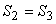 . .
- 3-4, reversible isothermal heat transfer
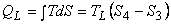 area 3-4-a-B. area 3-4-a-B.
- 4-1, reversible adiabatic compression isentropic process,
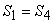 . .
- Net work,
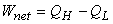 the area enclosed by 1-2-3-4, the shaded area. the area enclosed by 1-2-3-4, the shaded area.
We have also done a Second Law Analysis of the Flow processes (control volume based method)
|