| |
TdS Equations
- For a closed system contaning a pure compressible substance undergoing a reversible process.
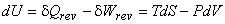
|
(24.22) |
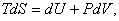 or or 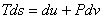 (per unit mass) (per unit mass) |
(24.23) |
This is the famous Gibbsian equation.
- Eliminate du by using the definition of enthalpy
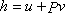
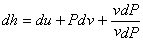 |
(24.24) |
Thus,
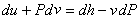 |
(24.25) |
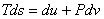 |
(24.26) |
Also,
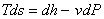 |
(24.27) |
- Important: These equations relate the entropy change of a system to the changes in other properties :
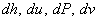 . Therefore, they are independent of the process . . Therefore, they are independent of the process .
These relations can be used for reversible as well as irreversible processes.
|