| |
Refrigerants
In the vapour compression refrigeration system, the working fluid undergoes a phase change during the cycle. There are a few other requirements. The first requirement is that the pressure at which the refrigerant enters the compressor should be slightly higher than the atmospheric pressure to make sure that no air leaks in. It is also necessary that the boiling point of the refrigerant should be lower then the temperature at which the evaporator is to be maintained. The refrigerant is compressed to a pressure of approximately 1 MPa where its saturation temperature is greater than the surrounding temperature so that energy can be rejected to the surroundings. During the energy rejection process the refrigerant undergoes a phase change from vapour to liquid. Hence the refrigerant's critical pressure should be high. The refrigerant should be non-toxic and non flammable. In view of this the commonly used refrigerant are Ammonia and Freons. Generally Freons are methane-based hydrocarbons where hydrogen atoms are replaced by chlorine or fluorine atoms. These are denoted by a number of two digits, where the first digit minus one is the number of hydrogen atoms and the second digit indicates the number of fluorine atoms, while the other atoms are chlorine.
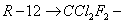 dichloro – difluoro methane dichloro – difluoro methane |
|
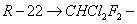 monochloro –difluorp methane monochloro –difluorp methane |
|
|