Mixture of ideal gases
Basic assumption is that the gases in the mixture do not interact with each other.
Consider a mixture with components l = 1,2,3... with masses m1, m2, m3 ...mi and with 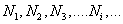 number of moles. number of moles.
The total mixture occupies a volume V, has a total pressure P and temperature T (which is also the temperature of each of the component species)
The total mass
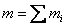 |
(34.1) |
Total number of mole N
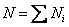 |
(34.2) |
Mass fraction of species i
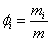 |
(34.3) |
Mole fraction of species i
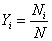 |
(34.4) |
The mass and number of moles of species i are related by
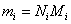 |
(34.5) |
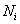 is the number of moles of species i and is the number of moles of species i and 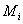 is the molar mass of species i is the molar mass of species i
Also to be noted
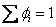 and and 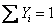 |
(34.6) |
We can also define a molar mass of the mixture as
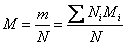 |
(34.7) |
or,
or,
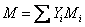 |
(34.8) |
|