Dalton 's Law of partial pressure
Total pressure of an ideal gas mixture is equal to the sum of the partial pressures of the constituent components, That is
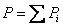 |
(34.9) |
P is the total pressure of the mixture
Pi is the partial pressure of species i
= pressure of the species if it existed alone in the given temperature T and volume V
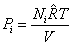 |
(34.10) |
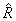 is the universal gas constant = 8.314 kJ/k mol K is the universal gas constant = 8.314 kJ/k mol K
Dalton 's Law
or
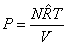 |
(34.11) |
The pressure function of species i
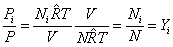 |
(34.12) |
Therefore, Pressure fraction = Mole fraction
|