Therdynamic Properties of Mixtures
Internal Energy
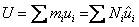 |
(34.17) |
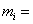 mass of species i mass of species i
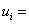 specific internal energy of species i specific internal energy of species i
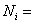 number of moles of species i number of moles of species i
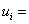 molar internal energy molar internal energy
Similarly we can write about Enthalpy
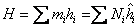 |
(34.18) |
Entropy
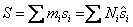 |
(34.19) |
Specific internal energy of the mixture
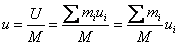 |
(34.19) |
or
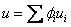 |
(34.20) |
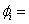 mass fraction of species i mass fraction of species i
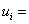 specific internal energy of species i specific internal energy of species i
Molar internal energy of mixture
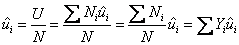 |
(34.21) |
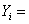 mole fraction of species i mole fraction of species i
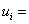 molar internal energy of species i molar internal energy of species i
Similarly we can write for specific enthalpy and molar enthalpy
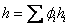 and and 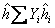 |
(34.22) |
We can also write for specific entropy and molar entropy
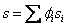 and and 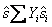 |
(34.23) |
Change in u, h and s
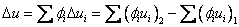
|
|
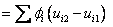 |
(34.24) |
Assume 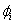 remains unchanged remains unchanged
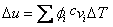 |
(34.25) |
For ideal gas mixture
as 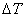 is the same for all species as a result of thermodynamics equilibrium is the same for all species as a result of thermodynamics equilibrium
or
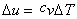 |
(34.26) |
Where 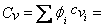 definition of mixture definition of mixture 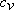
Similarly it can be shown that
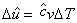 |
(34.27) |
Where
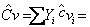 mixture mixture 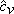 (molar basis) (molar basis) |
|
Let us also recall that 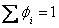 and and 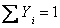
We can also write similar relations for mixture enthalpy
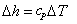 |
(34.28) |
Where
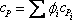 definition of mixture definition of mixture 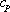 |
|
and
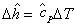 |
(34.29) |
Where
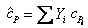 definition of mixture definition of mixture 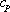 (molar basis) (molar basis) |
|
Therefore, 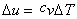 and and 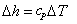 . The equations are similar to the equations for a single (ideal gas) species. . The equations are similar to the equations for a single (ideal gas) species.
|